Consider dissolving these two molecules in water: Even though the solute-separation step for ethanol requires more energy
Question:
Consider dissolving these two molecules in water:
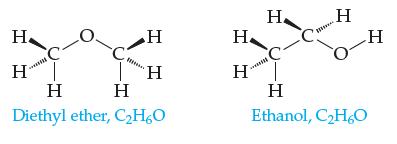
Even though the solute-separation step for ethanol requires more energy than does the solute-separation step for diethyl ether, ethanol is more soluble in water. Explain why.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





