Consider the basic hydrolysis (reaction with aqueous base) of (CH3) 3 CBr. The rate law is first
Question:
Consider the basic hydrolysis (reaction with aqueous base) of (CH3)3CBr.
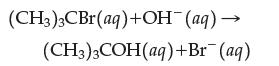
The rate law is first order with respect to (CH3)3CBr and zero order with respect to OH–. What does this imply about the mechanism of this reaction?
Transcribed Image Text:
(CH3)3CBr(aq) +OH¯(aq) → (CH3)3COH(aq) + Br (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The fact that the rate law for the basic hydrolysis of CH33CBr is first order with respect to CH33CB...View the full answer

Answered By

Lisper Wanja
I am an experienced and highly motivated writer with a passion for the skills listed. I have a proven track record of my expertise and my aim is to deliver quality, well-detailed and plagiarism free projects. My genuine passion for writing combined with my ongoing professional development through school and research makes me an ideal candidate within for any assignment.
4.90+
233+ Reviews
388+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Consider a teenager who evaluates whether she should engage in sexual activity with her partner of the opposite sex. She thinks ahead and expects to have a present discounted level of life-time...
-
The decomposition reaction of N2O5 in carbon tetrachloride is 2 N2O5 -- 4 NO2 + O2. The rate law is first order in N2O5. At the rate constant is 4.82 10-3 s-1. (a) Write the rate law for the...
-
Consider a simple time series model where the explanatory variable has classical measurement error: where ut has zero mean and is uncorrelated with x*t, and et. We observe yt and xt only. Assume that...
-
In which section do you create VLAN on Cisco WLC ? ? Layer 3 3 Section Layer 2 2 Section Security Section RF Section
-
Briefly explain what is meant by transaction analysis. What are the two steps in transaction analysis?
-
Suppose you won the lottery and had two options: (1) receiving $0.5 million or (2) taking a gamble in which at the flip of a coin you receive $1 million if a head comes up but receive zero if a tail...
-
Two of the wavelengths emitted by a hydrogen atom are \(102.6 \mathrm{~nm}\) and \(1876 \mathrm{~nm}\). a. What are the Balmer formula \(n\) and \(m\) values for each of these wavelengths? b. For...
-
Direct your attention to the company with perhaps the largest private pension plan in the worldGeneral Motors. GMs note relating to its pension plan is included in Exhibit 17-11 on pages 10561057....
-
During 2017, Blame had the following transactions: Salary $50,000 Interest on Treasury Bonds $1,000 Repayment received on a loan made to cousin several years ago $2,000 Life insurance proceeds...
-
Consider the decomposition of ozone (O 3 ) to oxygen (O 2 ). The rate law for this reaction is: Rate = k[O 3 ] 2 /[O 2 ]. How is the rate of this reaction affected by the concentration of oxygen?...
-
Consider the decomposition reaction of ozone into oxygen: 2O 3 (g) 3O 2 (g) Suppose the mechanism for this reaction is just the collision between two ozone molecules, as shown in the following...
-
For its numerator, the MAD statistic uses the sum of absolute pairwise differences. The absolute values get rid of possible negative differences. Instead of taking absolute values, what is another...
-
Both marketing and marketing communication are changing rapidly. New tools and methods are coming up and especially due t o changes in technology and the consumers themselves. Despite all these...
-
Discuss a situation where communication is important. This can be in your daily life, your work life, or in your academic life. Describe your own personal communication style preferences in the...
-
A high voltage resistive divider has bandwidth of 75 MHz. Can this divider be used for monitoring voltage signals shorter than 1.5 ns? Justify your answer?
-
Provide practical tips and strategies to improve communication with individuals with hearing disabilities. This could include using visual aids, gestures, and facial expressions, using assistive...
-
You will be acting as an external communication consultant for Starbucks. In your group you will discuss effective team-building strategies using the textbook as a guide and reference. In addition,...
-
Merlene owns a bookstore. The store needs repainting, but she is short of cash to hire a painter. Fred is a painter who enjoys fine mystery novels. Merlene makes a deal with Fred to have him paint...
-
Differentiate the following terms/concepts: a. Personality types and money attitudes b. Planners and avoiders c. Moderating and adapting to biases d. "Perfectible judges" and "incorrigible judges"
-
Pulling gs. Suppose again you are the astronaut in Problem 9. When most people are subjected to an acceleration greater than about 5 g, they will usually become unconscious (black out). Will you be...
-
Consider a string with one end tied to a tall ceiling and the other end hanging freely. Explain why the tension at the bottom of the string is smaller than the tension at the top.
-
Draw a qualitative plot of the total force acting on the ball in Figure 3.15 (page 68) as a function of time. Begin your plot while the ball is still in the throwers hand and end it after the ball...
-
Oh, no! The television was finally delivered today, but was left on the porch by the delivery company. When Jamie Lee was finally able to attach all the wires and cables according to the owners...
-
You are about to rent your first apartment. You have approximately $10,000 worth of personal belongings. Contact an insurance agent to find out the cost of renters insurance.
-
To save on future home insurance costs, you need a new roof and a burglar alarm system that will cost $10,000 five years from now. You can earn 3 percent on your savings. How much should you deposit...

Study smarter with the SolutionInn App


