Consider the following reaction energy profile: (a) At a given temperature, which is faster, the forward reaction
Question:
Consider the following reaction energy profile:
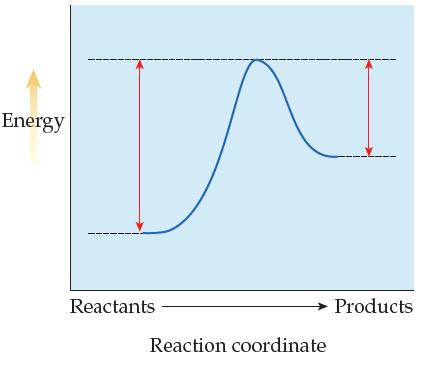
(a) At a given temperature, which is faster, the forward reaction or the reverse reaction? Explain how you knew.
(b) Based on your answer to (a), is kf > kr or kr > kf? Explain.
(c) Does this reversible reaction reach equilibrium to the left or right of center? Explain how you knew.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





