Consider the ground-state beryllium (Be) and carbon (C) atoms, shown below. (a) Indicate which atom is smaller
Question:
Consider the ground-state beryllium (Be) and carbon (C) atoms, shown below.
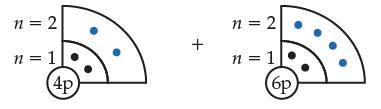
(a) Indicate which atom is smaller and explain why.
(b) Which ground-state atom has valence electrons that look like dumbell-shaped clouds? Explain how you knew this.
Transcribed Image Text:
n = 2 n = 1 4p + n = 2 n = 1 (6p)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a The beryllium atom is smaller than the carbon atom This is because the beryllium atom has a smaller nuclear charge The nuclear charge is the force t...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Your home business uses 570 square feet of your 2,850 square foot home. If household expenses for the year were $28,558, how much was alloted to your business? Amount invested
-
Newport Beach Manufacturing Corporation uses a standard cost system that records raw materials at actual cost, records materials price variances at the time that raw materials are issued to work in...
-
Anna Schaub, the financial manager at the Mangiamo restaurant, is checking to see if there is any relationship between newspaper advertising and sales revenues at the restaurant. She obtains the...
-
Consider the following cash flow profile, and assume MARR is 10 percent/year and the finance rate is 4 percent/year. a. Determine the MIRR for this project. b. Is this project economically...
-
Hawthorn Corporations adjusted trial balance contained the following accounts at December 31, 2012: Retained Earnings $120,000; Common Stock $750,000; Bonds Payable $100,000; Paid-in Capital in...
-
A 15 kg box is given a shove to the right on a horizontal, rough surface. After the hand loses contact, the box's acceleration (using standard directions) is a = (-1.2 m/s, 0). (a) What are the...
-
Explain what the following diagram has to say about where one might find lithiums valence electron. 2s
-
Regarding the nth period element whose position is indicated below with an X, which statement(s) are correct? (a) The element is a representative nonmetal. (b) The element is a transition metal. (c)...
-
For each of the scenarios identified in the following table, briefly explain in which countrys GDP and GNP the transaction is recorded. Scenario Roberto is a Mexican citizen who is an economics...
-
Beta Corporation is making plans for the next fiscal year. Betas current sales are $3.5 million, expected to increase to $5 million, based on current assets of $2.4 million and fixed assets of $2.5...
-
Design another experiment using difference-indifferences to understand the effect of a policy change at your college.
-
The Daher Trucking Company needs to expand its fleet by 20 percent to meet the demands of two major contracts it just received to transport aeronautic equipment from manufacturing facilities...
-
Garr Company estimates its investment to be $0.25 in assets for each dollar of new sales. By each dollar of additional sales $0.04 profits will be produced and $0.01 can be reinvested in the company....
-
Grays Accounting pays Rita Flores $ 51,000 per year. Flores works 1,000 hours per year. Requirements 1. What is the hourly cost to Gray Accounting of employing Flores? Assume a 25-hour week and a...
-
As indicated in the chapter, ROI is well entrenched in business practice. However, its use can have negative incentive effects on managerial behavior. For example, assume you are the manager of an...
-
A business had revenues of $280,000 and operating expenses of $315,000. Did the business (a) Incur a net loss (b) Realize net income?
-
Use the anharmonic potential function in Figure 18.7 to demonstrate that rotation and vibration are not separable degrees of freedom for large quantum numbers. Figure 18.7 (X)A
-
Conservation of energy requires that the variation of the potential and kinetic energies with the oscillator extension be exactly out of phase. Explain this statement.
-
What is the degeneracy of the energy levels for the rigid rotor in two dimensions? If it is not 1, explain why.
-
A hedge fund charges an incentive fee of 15% of any investment returns above the T-bill rate, which currently is 3.0% but is subject to a high water mark. In the first year, the fund suffers a loss...
-
R. v. Haevischer, 2023 SCC 11. This is a case dealing with the police conduct and conditions of pre-trial confinement which the accused believed amountd to an abuse of process. Who wrote the judgment...
-
Grandin Inc. is evaluating its dividend policy. It has a capital budget of $625,000, and it wants to maintain a target capital structure of 60% debt and 40% equity. The company forecasts a net income...

Study smarter with the SolutionInn App


