Consider the reaction Kinetics studies reveal a first-order rate dependence on the concentration of the (CH 3
Question:
Consider the reaction
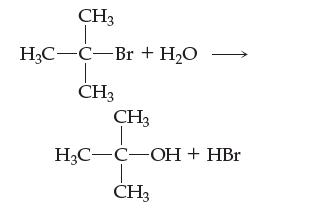
Kinetics studies reveal a first-order rate dependence on the concentration of the (CH3)3C—Br and a zero-order dependence on the concentration of H2O.
(a) What happens to the reaction rate as the (CH3)3C—Br concentration is changed? What happens to the reaction rate as the H2O concentration is changed?
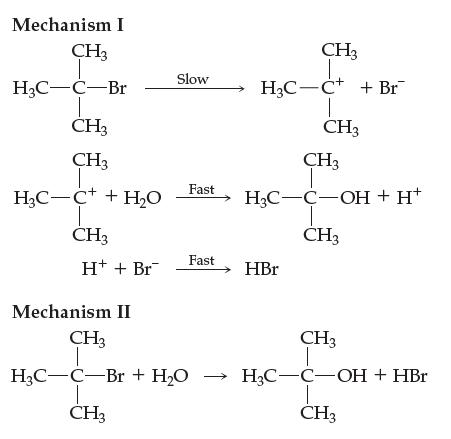
(b) Two mechanisms for this reaction are offered below. Can you rule out either of them? Is either mechanism plausible, given the overall balanced equation and kinetic data? Explain your answer fully.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





