Given a reaction vessel with the initial conditions shown on the next page, which set of rate
Question:
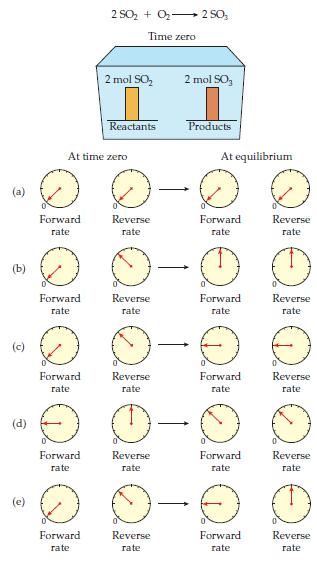
Given a reaction vessel with the initial conditions shown on the next page, which set of rate meters best describes the time-zero and equilibrium conditions? Explain why your choice is correct and why the other choices are incorrect.
Transcribed Image Text:
다 Forward rate D 2 SO₂ + O₂ 2 50₂ Time zero At time zero Forward rate 2 mol SO₂ Reactants Forward rate Reverse rate rate -2 0-0 0 Forward Reverse Reverse rate rate Reverse rate rate 2 mol SO, Reverse rate Products - - At equilibrium Forward rate -2 0-0 Forward Reverse rate Forward. rate Forward rate Forward rate Reverse rate Forward rate Reverse rate Reverse rate Reverse rate
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a Is incorrect the reverse rate must be fast at time zero because the vessel is ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
You are the CFO of your organization where you will be deciding between three choices of consolidation. This assignment will ask you to select a choice that best supports short and long-term goals of...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Case Study: Quick Fix Dental Practice Technology requirements Application must be built using Visual Studio 2019 or Visual Studio 2017, professional or enterprise. The community edition is not...
-
Redo Exercise 29 for the situation in which Ms. Jones withdrew $1000 at the end of the seventh year instead of depositing it. Data in Exercise 29 Ms. Jones deposited $100 at the end of each month for...
-
Kelvins Commemoratives makes and sells two types of decorative plates. One plate displays a hand-painted image of Princess Diana; the other plate displays a machine-pressed image of Marilyn Monroe....
-
A 2.5 kg block is initially at rest on a horizontal surface. A horizontal force F of magnitude 6.0 N and a vertical force P are then applied to the block (Figure). The coefficients of friction for...
-
How much current is drawn by the lamp at the outlet? That is, what is the rms current in the primary? A. \(0.42 \mathrm{~A}\) B. \(1.3 \mathrm{~A}\) C. \(4.2 \mathrm{~A}\) D. \(13 \mathrm{~A}\)...
-
For the year ending December 31, 2014, Cobb Company accumulates the following data for the Plastics Division which it operates as an investment center: contribution margin $700,000 budget, $710,000...
-
Revenues Costs and expenses Operating income Other income (expense)* $ 39,588 38,165 1,423 (77) Income before income taxes 1,346 683 $ 663 Income tax expense Net income *Includes $188 of interest...
-
Starting only with reactants, a reaction can be said to have reached equilibrium when: (a) The concentration of reactants no longer decreases. (b) The concentration of products no longer increases....
-
The equilibrium constant for the reaction N 2 O 2 (g) N2(g) + O 2 (g) is 0.456 at 322 k. Suppose we put 1.00 mole of N 2 O 2 into a 1.00-L container and heat it to 322 k. At time zero, the...
-
Name and describe the three basic mechanisms of heat transfer on Earth, their relative rates of heat transfer, and the part of the global environment in which each is most active.
-
State the characteristic feature of random sample selection and list three variations of random sample selection.
-
Use the Divergence Theorem to evaluate the flux \(\iint_{\mathcal{S}} \mathbf{F} \cdot d \mathbf{S}\). \(\mathbf{F}(x, y, z)=\left\langle x^{2} z, y x, x y zightangle, \mathcal{S}\) is the boundary...
-
Explain briefly how auditing techniques have been affected by: (i) growth in the size of companies (ii) changes in technology.
-
(a) Give examples of resources that might be treated as assets in a balance sheet but normally are not. How helpful is the Framework definition of assets in making clear that they are not included?...
-
Suppose that the two nations in Problems 31-1 and 31-2 choose to specialize in producing the goods for which they have a comparative advan- tage. They agree to trade at a rate of exchange of 1 pastry...
-
Explain relationships between the Rehabilitation Act of 1973 and the Americans with Disabilities Act.
-
In Exercises, find the equation of the tangent line at the given point on each curve. 2y 2 - x = 4; (16, 2)
-
A pole vaulter of mass 70 kg can run horizontally with a top speed of 10.0 m/s. The current record height for the pole vault is about 6.2 m. (a) For the vaulter to clear this height, approximately...
-
A bomb that is initially at rest breaks into several pieces of approximately equal mass, two of which are shown with their velocity vectors in Figure Q7.1. Use conservation of momentum to determine...
-
A toy gun shoots spherical plastic projectiles by means of a spring. A typical projectile has mass m = 25 g. The spring used has spring constant k = 15 N/m, and when put under load, it is displaced...
-
Read the article "16 Firms Worth Billions Despite Losing Money." http://money.cnn.com/2015/01/23/investing/shazam-tech-startups-lose- money/index.html Discussion terms: a) As of August 2017, Twitter...
-
What is an individual who receives Medicare referred to as for insurance purposes?
-
Ken Barker received a $10,000 scholarship to study undergraduate engineering at Rainbow University for the current academic year. Ken received the $10,000 in cash, and he used the money as follows:...

Study smarter with the SolutionInn App


