Here are data for three hypothetical reactions: For each reaction, (a) Sketch the reaction-energy profile. (b) Indicate
Question:
Here are data for three hypothetical reactions:
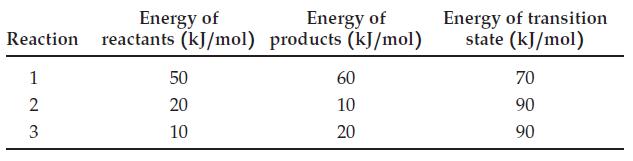
For each reaction,
(a) Sketch the reaction-energy profile.
(b) Indicate whether the reaction is endothermic or exothermic.
(c) Determine the value of ΔErxn.
(d) Determine the value of Ea.
Transcribed Image Text:
Energy of Energy of Reaction reactants (kJ/mol) products (kJ/mol) 1 23 2 50 20 10 60 10 20 Energy of transition state (kJ/mol) 70 90 90
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
a b Reaction 1 endothermic ...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
The rates of many atmospheric reactions are accelerated by the absorption of light by one of the reactants. For example, consider the reaction between methane and chlorine to produce methyl chloride...
-
The energy profile diagram represents (a) An endothermic reaction (b) An exothermic reaction (c) A fast reaction (d) A termolecular reaction A + B Progress of reaction >
-
Indicate whether each statement is true or false. If it is false, rewrite it so that it is true. (a) If you measure the rate constant for a reaction at different temperatures, you can calculate the...
-
The stockholders' equity accounts of Whispering Company have the following balances on December 31, 2025. Common stock, $10 par, 304,000 shares issued and outstanding $3,040,000 Paid-in capital in...
-
Preparing an Income Statement, Statement of Retained Earnings, and Balance Sheet Assume that you are the president of Influence Corporation. At the end of the first year (June 30, 2011) of...
-
William Bryant is the new owner of Ace Computer Services. At the end of August 2012, his first month of ownership, Bryant is trying to prepare monthly financial statements. Below is some information...
-
Select a publicly traded company for analysis. Using the firms most recent 10-K Report, accessed through the SEC EDGAR database at sec.gov or from the companys website, analyze the firms Statement of...
-
1. What policies and procedures does Whole Foods enact that allows it to develop successful associate teams? 2. What impact do you think that the process of allowing team members to vote on hiring...
-
What journal entries would Wonka Inc. make to repurchase the $1,000,000 outstanding bonds with a 5% stated interest rate at 80% of the face value and replace them with a new issue of $1,000.000 of...
-
A reaction is endothermic by 65 kJ/mol (that is, E rxn = +65 kJ for every mole of product formed). (a) Draw a reaction-energy profile for this reaction, assuming the energy of the reactants to be 100...
-
Kinetics studies are often performed for the following reason: (a) To determine the purity of the products of the reaction. (b) To give insight into the mechanism of the reaction (how it occurs at...
-
A gaseous mixture consists of 80.0 mole percent N2 and 20.0 mole percent O2 (the approximate composition of air). Suppose water is saturated with the gas mixture at 25C and 1.00 atm total pressure,...
-
Are there any limitations or barriers to this process?(Kotter's eight steps). Define and also the eight steps
-
What is the worst complication that the patient may experience, and how will I prevent, identify and treat Type I Diabetes Mellitus complication? (Death is the worst, but what will cause it)
-
What defines an organic culture? Low accountability Specific processes Employee accountability for decision making Decisions guided by policy
-
"A company's revenue depends on the amount of effort the sales team puts in. The sales team can possibly be incentivized to put in higher effort if the company pays a commission on each sale made."...
-
Under the temporal method, cost of goods sold (COGS) in foreign currency (FC) is translated into parent company currency by Multiple choice question. multiplying COGS in FC by the end-of-period...
-
Dales son is a senior in high school and is planning to go away to college next year. Dale would like to buy a house near the campus and rent it to his son. He comes to you for advice on the tax...
-
The age-old saying for investing is "buy low and sell high," but this is easier said than done. Investors who panic about falling prices sell their investments, which in turn lowers the price and...
-
Soccer on a windy day. A professional soccer player can kick a ball with a speed of about 30 m/s (about 75 mi/h). Does air drag play a significant role in the trajectory of the ball? Give a reason...
-
When airplanes land or take off, they always travel along a runway in the direction that is into the wind because the lift force on an airplane wing depends on the speed of the airplane relative to...
-
A projectile is fired uphill as sketched in Figure P4.90. If v 0 = 150 m/s and θ = 30°, what is L? Figure P4.90 0 = 30
-
Proposal Annual cost of capital required Variable cost of each component One: purchase $0.00 $26.50 Two: make with rebuilt equipment $125,000.00 $16.50 Three: make with new equipment $425,000.00...
-
The company is associated with one other company. Maximum's share of the limit is $375,000. The associated company has no AAII for either of 2021 or 2022. Required: A. Determine Maximum's 2022 small...
-
Aaron Company completed 11,800 units of its single product, consuming 36,000 labor hours that cost the firm $554,400. Each unit should have required 3 hours of labor time at $15.80 per hour. On the...

Study smarter with the SolutionInn App


