Here is a diagram of a battery made from nickel and lead: (a) In the diagram above,
Question:
Here is a diagram of a battery made from nickel and lead:
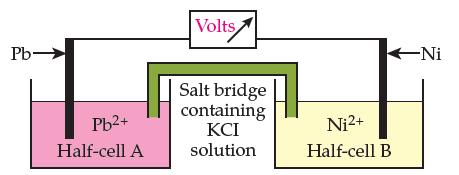
(a) In the diagram above, label the anode and the cathode and draw an arrow above the voltmeter to show the direction of the electron flow.
(b) Write the net ionic equation for the spontaneous reaction that occurs in this battery.
(c) In which half cell does reduction take place?(Circle one.)
Reduction occurs in the Pb half cell
Reduction occurs in the Ni half cell
(d) Into which half cell will the potassium (K+) ions in the salt bridge flow? (Circle one.)
The Pb half cell
The Ni half cell
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





