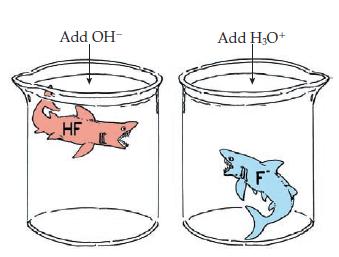
Imagine a buffer made by combining an aqueous solution of the weak acid HF with its sodium
Question:
Imagine a buffer made by combining an aqueous solution of the weak acid HF with its sodium salt, NaF.
(a) Write the reaction responsible for replacing any added strong base (OH–) by a weak base.
(b) Write the reaction responsible for replacing any added strong acid (H3O+) by a weak acid.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





