In a kinetic study of the reaction the following rate data were obtained. Write a rate law
Question:
In a kinetic study of the reaction
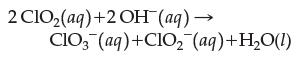
the following rate data were obtained. Write a rate law complete with proper values for the orders. What is the overall order of the reaction?![Experiment 1 23 2 [C10] 0.060 M 0.020 M 0.020 M [OH-] 0.030 M 0.030 M 0.090 M Rate (M/s) 0.024 84 0.002 76](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/8/5/9/10265704f1e898191701859100897.jpg)
Transcribed Image Text:
2 C10₂(aq)+ 2 OH (aq) → ClO3(aq)+CIO₂ (aq) +H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To determine the rate law for the given reaction and the overall order you can use the provided rate ...View the full answer

Answered By

Lucy Lagamayo
I taught Japanese professionals online as ESL teacher and as Assistant Language Teacher in a public school in Japan.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
In a kinetic study of the reaction 2A(g) + B(g) P(g) the following rate data were obtained. Write the rate law with proper orders. Give the overall order of the reaction. Finally, state what this...
-
In a kinetic study of the reaction 2NO(g) + O2(g) 2NO2(g) the following data were obtained for the initial rates of disappearance of NO: Obtain the rate law. What is the value of the rate constant?...
-
Consider the following reaction between mercury (II) chloride and oxalate ion: 2 HgCI2(aq) + C2O42- (aq) 2 CIË (aq) + 2 CO2(g) + Hg2CI2(s) The initial rate of this reaction was determined for...
-
True or False? Azure storage is used by both Infrastructure as a Service ( ( laaS ) ) virtual machines, and Platform as a Service ( ( PaaS ) ) cloud services. True False
-
Determining Financial Statement Effects of Adjusting Entries Refer to P4-3. Required: 1. Indicate whether each transaction relates to a deferred revenue, deferred expense, accrued revenue, or accrued...
-
Lloyd Inc. has sales of $200,000, a net income of $15,000, and the following balance sheet: The new owner thinks that inventories are excessive and can be lowered to the point where the current ratio...
-
Carbon is the sixth element in the periodic table. How many protons and how many neutrons are there in a nucleus of the isotope 14 C?
-
1. What were Bakers intentions in the conversation with Rennalls? Were they fulfilled or not, and why? 2. Was Baker alert to nonverbal signals? What did both Baker and Rennalls communicate to one...
-
Sage Hill Inc. owns 25% of the common shares of Sheffield Corp. The other 75% of the shares are owned by the Sheffield family. Sage Hill acquired the shares eight years ago through a financing...
-
Does the following reaction-energy profile represent an endothermic or exothermic reaction in the forward direction? In the reverse direction? Energy, kJ/mol 200 100- 0 -100 -200 Reactants Start...
-
A student measures the rate of a reaction by measuring how much the concentration of one of the reactants changed over a period of 5 s. She writes in her lab book that the reaction rate is 2.00 10 2...
-
Evaluate the expression. Approximate the answer to the nearest hundredth when appropriate. 8 1/3
-
How would you design a compensation/benefits program to appeal to generational differences in the workplace? How would you design a rewards program to appeal to generational differences in the...
-
If you were the administrator of a small (150-200 bed) skilled nursing home what are some things that you would do to create a culture of safety and quality.
-
Effective human resource management and organizational behavior as they relate to both Fair treatment and legal compliance
-
Which qualitative and quantitative metrics would be recommended to evaluate compensation initiatives at an organization like Walmart?
-
What are the differences between the Cafeterya - style and Total Rewards? Text citation What are the aims of Pay and Rewards? Text Citation
-
Hermano and Rosetta are a retired couple who receive $10,000 in Social Security benefits during the current year. They also receive $3,000 in interest on their savings account and taxable pension...
-
Federated Shipping, a competing overnight delivery service, informs the customer in Problem 65 that they would ship the 5-pound package for $29.95 and the 20-pound package for $59.20. (A) If...
-
Two rocks are thrown off a cliff. One rock (1) is thrown horizontally with a speed of 20 m/s. The other rock (2) is thrown at an angle relative to the horizontal with a speed of 30 m/s. While the...
-
A ball is thrown straight up and rises to a maximum height of 24 m. At what height is the speed of the ball equal to half its initial value? Assume the ball starts at a height of 2.0 m above the...
-
Consider the problem of kicking a soccer ball past a goalkeeper into the goal (Fig. P4.29). You are 25 m away from the goal and kick the ball at an angle of 30° with respect to the horizontal,...
-
Use an Internet website to locate the current price for a share of stock and earnings per share for Microsoft (symbol MSFT), 3M Company (symbol MMM), and Colgate-Palmolive (symbol CL).
-
Jamie Lees father suggested that they purchase stock in a company that he has held shares in for decades.question They want to take advantage of the stock tip, but Jamie Lee and Ross are trying to...
-
Suppose Cute Camel Woodcraft Company is evaluating a proposed capital budgeting project (project Alpha) that will require an initial investment of $400,000. The project is expected to generate the...

Study smarter with the SolutionInn App


