In the pie-chart graphs, the percent natural abundance for Cl-35 (atomic mass 34.969 amu) is represented as
Question:
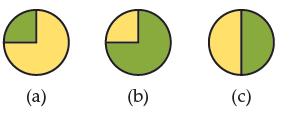
In the pie-chart graphs, the percent natural abundance for Cl-35 (atomic mass 34.969 amu) is represented as green, and the percent natural abundance for Cl-37 (atomic mass 36.966 amu) is represented as yellow. Given that the atomic mass listed for Cl in the periodic table 35.353 amu, and without looking up the percent natural abundances for either isotope, which pie chart is most correct? Explain your reasoning.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





