Of the three solutions shown here, (a) Which is a weak electrolyte, which is a strong electrolyte,
Question:
Of the three solutions shown here,
(a) Which is a weak electrolyte, which is a strong electrolyte, and which is a nonelectrolyte? How can you tell?
(b) Which of these compounds is/are molecular? Which is/are ionic?
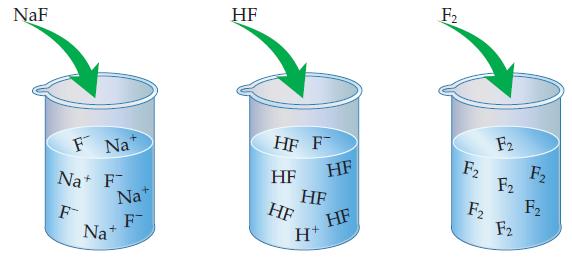
Transcribed Image Text:
NaF F Na Na+ F F Nat F Na+ HF HF F HF HF HF HF H+ HF F₂ F₂ F2 F2 F2 F2 F₂ F₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a NaF is a strong electrolyte because it dissociates completely HF i...View the full answer

Answered By

Shadrack Mulunga
I am a Biochemistry by profession. However, I have explored different fields of study. My quest to explore new fields has helped me gain new knowledge and skills in Business, clinical psychology, sociology, organizational behavior and general management, and Project Management. I count my expertise in Project management, in particular, creation of Work Break Down Structure (WBS) and use of Microsoft Project software as one of my greatest achievement in Freelancing industry. I have helped thousands of BSC and MSC students to complete their projects on time and cost-effectively using the MS Project tool. Generally, I find happiness in translating my knowledge and expertise to success of my clients. So far, i have helped thousands of students to not only complete their projects in time but also receive high grades in their respective courses. Quality and timely delivery are the two key aspects that define my work. All those who hired my services always come back for my service. If you hire my services today, you will surely return for more. Try me today!
5.00+
154+ Reviews
289+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
When a pure substance is placed in contact with water, there are three possible outcomes. The substance may do nothing that is, the substance does not dissolve and no visible change takes place. The...
-
Part 1 a. Ammonia, NH 3 , is a weak electrolyte. It forms ions in solution by reacting with water molecules to form the ammonium ion and hydroxide ion. Write the balanced chemical reaction for this...
-
Three beakers contain clear, colorless liquids. One beaker contains pure water, another contains salt water, and another contains sugar water. How can you tell which beaker is which? (No tasting...
-
As the traffic manager for ABC Electronics, you have been charged with the task of reducing shipping costs for a fast selling cable product that is sold by the pound. You have a very satisfactory...
-
Preparing a Balance Sheet and Analyzing Some of Its Parts Exquisite Jewelers is developing its annual financial statements for 2012. The following amounts were correct at December 31, 2012: cash,...
-
Find a 95% confidence interval for the proportion of all customers whose order is for more than $100. Then do this separately for each of three times of day.
-
Although the customer loyalty project at Petrie Electronics had gone slowly at first, the past few weeks had been fast-paced and busy, Jim Watanabe, the project manager, thought to himself. He had...
-
Assume the facts in E13-5 except that Matt Broderick Company has chosen not to accrue paid sick leave until used, and has chosen to accrue vacation time at expected future rates of pay without...
-
n Juods xi(yi - Bxi) = 0 n i=1 a) Step by step, solve for B (Hint: you may want to use the proof starting on pg 10 as your guide)
-
(a) Is the compound H 2 SO 4 molecular or ionic? (b) How did you decide on your answer to part (a)? (c) Based on your answer to part (a), would you call H 2 SO 4 an electrolyte or a nonelectrolyte?...
-
A substance is an electrolyte if: (a) It conducts electricity. (b) It can produce electricity. (c) An aqueous solution of it conducts electricity. (d) It is a neutral substance.
-
Describe the concepts of differentiation and integration. Is integration an attempt to do away with differentiation?
-
Examine two drawbacks and two benefits associated with ISO 9000 certification, delineating its implications within organizational frameworks.
-
Cam is really mindful that keeping on top their cash is really important and has been taking note of their cash balances but is confused as to why their online bank balance never seems to match with...
-
23 2. 2 === Complete the following equations: + + + i 2 22 26 = + i 2001 = + Hint: All you need to know to solve this problem is that i = -1.
-
You bring your friend to the Biomechanics Lab and tell them to stand naturally in a static position on the force plate. The force plate measures their mass at 78 kg and measures their right foot 21...
-
Project A costs $1,000, and its cash flows are the same in Years 1 through 10. Its IRR is 16%, and its WACC is 11%. What is the project's MIRR? Do not round intermediate calculations. Round your...
-
Briefly describe the kinds and characteristics of restaurants.
-
For each of the following transactions, indicate whether it increases, decreases, or has no effect on the following financial ratios: current ratio, debt-to-equity ratio, profit margin ratio, and...
-
A person is riding in a car traveling on a straight level road. What is the direction of the net force on the person if the car is (a) Speeding up, (b) Slowing down, (c) Has a constant speed?
-
Give examples of objects whose motion is described by the plots in Figure P2.20. Figure P2.20 Case 2 Case 3 Case 1
-
Two football players start running at opposite ends of a football field (opposite goal lines), run toward each other, and then collide at the center of the field. They start from rest and are running...
-
Anna Rehearse started her practice as a design consultant, Rehearse Design, on April 1, 2022. During the first month of operations, the business completed the following transactions: Received $25,000...
-
Utilizing our Franciscan Values, discuss why it is imperative for global managers to think ethically. What does it mean to be ethical? Why should companies be ethical? What is the incentive (or lack...
-
What must you consider when implementing a Balanced Scorecard? Discuss 1-3 actions to take and 1 - 3 actions to avoid to implement a Balanced Scorecard.

Study smarter with the SolutionInn App


