Suppose you have a bottle of acetic acid and a bottle of sodium acetate, CH 3 CO
Question:
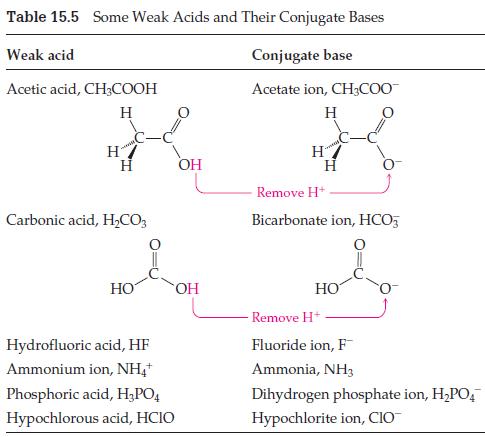
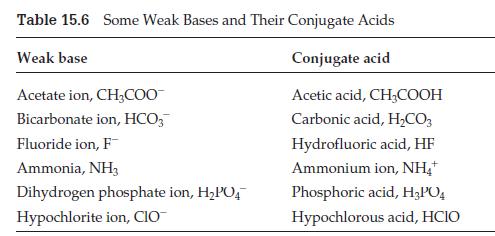
Suppose you have a bottle of acetic acid and a bottle of sodium acetate, CH3CO2Na, and you add some of each to the same beaker of water. Consult Tables 15.5 and 15.6 to help you answer the following questions.
(a) Is there a weak acid in the beaker? If so, what is it?
(b) Is there a weak base in the beaker? If so, what is it?
(c) Is there a conjugate pair in the beaker? If so, what is it?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





