Use these data to calculate the percentage of oxygen and hydrogen in these two samples of water:
Question:
Use these data to calculate the percentage of oxygen and hydrogen in these two samples of water:
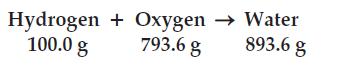
(a) Is the law of conservation of matter obeyed?
(b) What is the percent hydrogen in water?
(c) What is the percent oxygen in water?
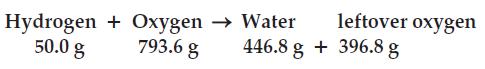
(d) Is the law of conservation of matter obeyed?
(e) What is the percent hydrogen in water?
(f) What is the percent oxygen in water?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





