A sample of sodium carbonate is treated with 50.0 mL of 0.345 M HCl. The excess hydrochloric
Question:
A sample of sodium carbonate is treated with 50.0 mL of 0.345 M HCl. The excess hydrochloric acid is titrated with 15.9 mL of 0.155 M NaOH. Calculate the mass of the sodium carbonate sample.
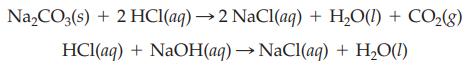
Transcribed Image Text:
Na₂CO3(s) + 2 HCl(aq) → 2 NaCl(aq) + H₂O(l) + CO₂(g) HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
078...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
An antacid tablet contains sodium hydrogen carbonate, NaHCO3, and inert ingredients. A 0.500-g sample of powdered tablet was mixed with 50.0 mL of 0.190 M HCl (hydrochloric acid). The mixture was...
-
An antacid tablet contains sodium hydrogen carbonate, NaHCO 3 , and inert ingredients. A 0.465-g sample of powdered tablet was mixed with 53.3 mL of 0.190 M HCl (hydrochloric acid). The mixture was...
-
A 6.53-g sample of a mixture of magnesium carbonate and calcium carbonate is treated with excess hydrochloric acid. The resulting reaction produces 1.72 L of carbon dioxide gas 28oC at and 743 torr...
-
You bought a share of 3.4 percent preferred stock for $96.82 last year. The market price for your stock is now $98.34. What is your total return for last year?
-
The trial balance of Dealer's Choice Wholesale Company contained the accounts shown at December 31, the end of the company's fiscal year. Adjustment data:1. Depreciation is $8,000 on buildings and...
-
Solve ln(2x + 1) < ln(x + 4).
-
When you remove a dielectric slab from between the plates of a charged isolated capacitor, what happens to the energy stored in the capacitor? Why does this happen to the stored energy?
-
Bill has just returned from a duck hunting trip. He has brought home eight ducks. Bills friend, John, disapproves of duck hunting, and to discourage Bill from further hunting, John has presented him...
-
Government spending as a fiscal policy tool is used to: A) ?Decrease the national debt B) ?Directly stimulate economic activity by increasing demand C) ?Reduce inflation D) ?Lower interest rates
-
What is the molar chloride ion concentration resulting from mixing of 50.0 mL of 0.100 M sodium chloride and 25.0 mL of 0.100 M potassium chloride?
-
What is the molar sodium ion concentration resulting from mixing of 50.0 mL of 0.100 M sodium chloride and 50.0 mL of 0.200 M sodium sulfate?
-
Dynamics Telecommunications Corp. has made an investment in another company that will guarantee it a cash flow of $22,500 each year for the next five years. If the company uses a discount rate of 15...
-
Suppose you have taken a years break mid-way through your career to pursue some social work. How would you mention this in your rsum?
-
Your interview in the previous case went well and you have been offered the job. However, there is a problem, and a new opportunity has presented itself. Your task: The opportunity to travel for a...
-
Creating presentations and other multimedia supplements can be a great way to expand on the brief overview that a rsum provides. Your task: Starting with any version of a rsum youve created for...
-
As a recruiter for a major multinational organization, you are impressed by a particularly interesting rsum and set up a screening interview with the applicant. During this interview, you discover...
-
The concept of video rsums and their increasing popularity means that an understanding of ideal content and practice in production is important for prospective employees. Your task: Plan and produce...
-
What is a non-value-adding activity?
-
State whether each statement is true or false. If false, give a reason. {purple, green, yellow} = {green, pink, yellow}
-
Soft Glow Candle Co. projected sales of 78,000 candles for 2010. The estimated January 1, 2010, inventory is 3,600 units, and the desired December 31, 2010, inventory is 4,500 units. What is the...
-
Day Timer Publishers Inc. projected sales of 205,000 schedule planners for 2010. The estimated January 1, 2010, inventory is 18,500 units, and the desired December 31, 2010, inventory is 15,000...
-
Soft Glow Candle Co, budgeted production of 78,900 candles in 2010. Wax is required to produce a candle. Assume 8 ounces (one half of a pound) of wax is required for each candle. The estimated...
-
Critical Analysis & Appraisal, Part I Record your responses in the space provided. The boxes will expand as you type. Note: This is your own critique. Your responses should be your own words and...
-
Fresh Foods established a petty cash fund of $275 on January 2. On January 31, the fund contained cash of $77.50 and vouchers for the following cash payments. Maintenance expense Office supplies...
-
Simon Company's year-end balance sheets follow. At December 31 Assets Current Year 1 Year Ago 2 Years Ago Cash Accounts receivable, net $ 34,681 98,506 $ 39,323 $ 43,939 73,072 55,741 Merchandise...

Study smarter with the SolutionInn App


