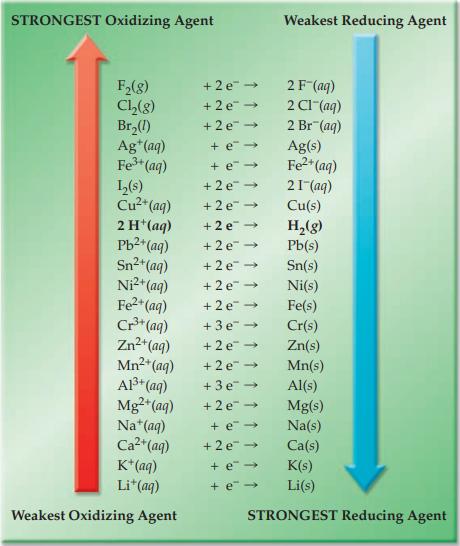
Refer to Figure 17.4 and indicate which substance in each of the following pairs has the greater
Question:
Refer to Figure 17.4 and indicate which substance in each of the following pairs has the greater tendency to be oxidized.
(a) Li(s) or K(s)
(b) Al(s) or Mg(s)
(c) Fe2+(aq) or I–(aq)
(d) Br–(aq) or Cl–(aq).
Figure 17.4
Transcribed Image Text:
STRONGEST Oxidizing Agent F₂(8) Cl₂(g) Br₂(1) Ag+ (aq) Fe³+ (aq) L₂(s) Cu²+ (aq) 2 H+ (aq) Pb²+ (aq) Sn²+(aq) Ni²+ (aq) Fe²+ (aq) Cr³+ (aq) Zn²+ (aq) Mn²+ (aq) Al³+ (aq) Mg²+ (aq) Na+ (aq) Ca²+ (aq) K+ (aq) Li+ (aq) Weakest Oxidizing Agent +2 e + 2e → +2e → + e → + e-> +2e → +2e →>> +2e → +2e → +2e → +2e → +2e → +3e → +2e → +2e → +3e → +2e → Weakest Reducing Agent + e→ +2e → + e→ + e → 2 F¯(aq) 2 Cl¯(aq) 2 Br (aq) Ag(s) Fe²+ (aq) 21-(aq) Cu(s) H₂(g) Pb(s) Sn(s) Ni(s) Fe(s) Cr(s) Zn(s) Mn(s) Al(s) Mg(s) Na(s) Ca(s) K(s) Li(s) STRONGEST Reducing Agent
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a Lis...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
According to general trends in the periodic table, predict which element in each of the following pairs has the greater metallic character. (a) K or Ca (b) Mg or Al (c) Fe or Cu (d) S or Ar. Periodic...
-
According to general trends in the periodic table, predict which element in each of the following pairs has the greater metallic character. (a) B or Al (b) Na or K (c) Mg or Ba (d) H or Fe. Periodic...
-
Refer to the periodic table and predict which element in each of the following pairs has the lower ionization energy. (a) Mg or Si (b) Pb or Bi (c) Ca or Ga (d) P or Cl. Periodic Table: 2 3 4 10 6 3...
-
At January 1, 2024, Mahmoud Industries, Inc., owed Second BancCorp $12 million under a 10% note due December 31, 2026. Interest was paid last on December 31, 2022. Mahmoud was experiencing severe...
-
Nike, Inc. reported the following plant assets and intangible assets for the year ended May 31, 2009 (in millions): other plant assets $965.8; land $221.6; patents and trademarks (at cost) $515.1;...
-
Find the function value. Round to four decimal places. tan 1086.2
-
Two horizontal parallel conducting rods are connected such that a conducting crossbar free to slide along them has a constant current \(I\) running through it (Figure P27.34). The rods are separated...
-
The J. Mehta Company's production manager is planning for a series of 1-month production periods for stainless steel sinks. The demand for the next 4 months is as follows: DEMAND FOR MONTH STAINLESS...
-
Your 3 year old nephew got into the workbench and mixed iron filings into a container with sugar and marbles. Explain the steps you would take to separate and recover each substance (use point form...
-
Chlorine can undergo a redox reaction in which it is simultaneously oxidized and reduced. Write a balanced equation for the following in a basic solution. Cl 2 (aq) ClO 2 (aq) + Cl (aq)
-
Chlorine can undergo a redox reaction in which it is simultaneously oxidized and reduced. Write a balanced equation for the following in an acidic solution. Cl 2 (aq) Cl (aq) + HOCl(aq)
-
True Or False A defamation plaintiff who suffers no quantifiable damages cannot go to trial.
-
Explain the circumstances in which return on investment is preferable as a measure of divisional performance.
-
Rather than a focus group, for this activity think about depth interviews with your friends. What questions would you ask them? Compare these questions to the focus group questions. How are they...
-
ACT Doorland was founded in 1957 as a small concern whose core business was the supply and installation of garage doors. Since then it has diversified its interests and expanded to become Canberras...
-
How do the managers of a business maintain its competitive position.
-
How does life-cycle costing help maintain competitive advantage.
-
Define the accounting rate of return.
-
How can NAFTA be beneficial to suppliers of Walmart?
-
Michelangelo Inc., a software development firm, has stock outstanding as follows: 20,000 shares of cumulative 1%, preferred stock of $25 par, and 25,000 shares of $100 par common. During its first...
-
On February 10, Peerless Rocks Inc., a marble contractor, issued for cash 40,000 shares of $10 par common stock at $34, and on May 9, it issued for cash 100,000 shares of $5 par preferred stock at...
-
On June 4, Magic Carpet Inc., a carpet wholesaler, issued for cash 250,000 shares of no-par common stock (with a stated value of $3) at $12, and on October 9, it issued for cash 25,000 shares of $75...
-
On January 1, 2021, Daniel Faust loaned $112,695 cash to Joyce Ladd in the form of a zero-interest-bearing note (face amount, $150,000). The note is to be repaid on December 31, 2023. The prevailing...
-
Grand Central Bakery is considering a buy one get one 50% off special for its croissants. Each croissant has a variable cost of $3.50 per item and currently sells for a retail price of $6. The fixed...
-
Rook Company sells video and board games. Below is last year's income statement for the board game segment: Sales $4,000,000 Variable expenses $2,600,000 Contribution margin $1,400,000 Fixed expenses...

Study smarter with the SolutionInn App


