Write a balanced net ionic equation for each of the following acidbase reactions. Refer to Table 14.5
Question:
Write a balanced net ionic equation for each of the following acid–base reactions. Refer to Table 14.5 and Appendix D for electrolyte information.
(a) HF(aq) + Li2CO3(aq) → LiF(aq) + H2O(l) + CO2(g)
(b) H2SO4(aq) + Ba(OH)2(aq) → BaSO4(s) + H2O(l)
Table 14.5
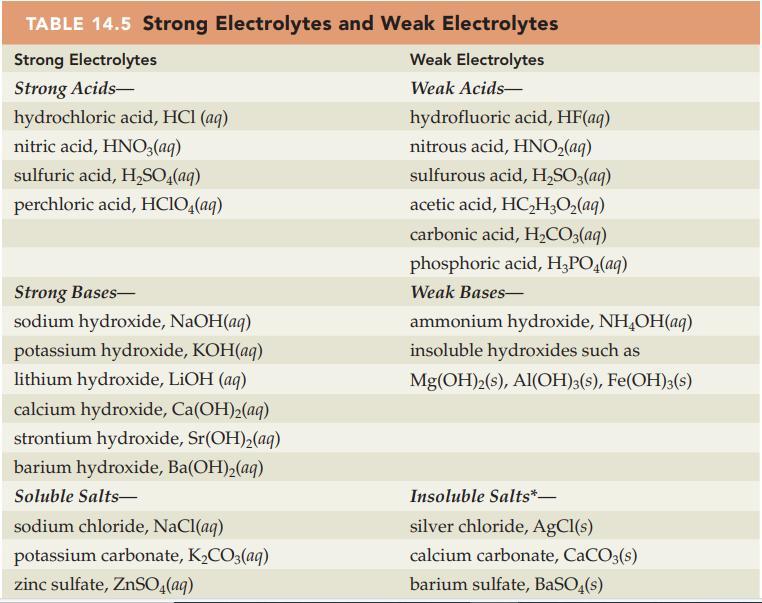
Transcribed Image Text:
TABLE 14.5 Strong Electrolytes and Weak Electrolytes Strong Electrolytes Weak Electrolytes Strong Acids- Weak Acids- hydrochloric acid, HCl (aq) nitric acid, HNO3(aq) sulfuric acid, H₂SO4(aq) perchloric acid, HClO4(aq) Strong Bases- sodium hydroxide, NaOH(aq) potassium hydroxide, KOH(aq) lithium hydroxide, LiOH (aq) calcium hydroxide, Ca(OH)₂(aq) strontium hydroxide, Sr(OH)₂(aq) barium hydroxide, Ba(OH)₂(aq) Soluble Salts- sodium chloride, NaCl(aq) potassium carbonate, K₂CO3(aq) zinc sulfate, ZnSO4(aq) hydrofluoric acid, HF(aq) nitrous acid, HNO₂(aq) sulfurous acid, H₂SO3(aq) acetic acid, HC₂H₂O₂(aq) carbonic acid, H₂CO3(aq) phosphoric acid, H₂PO4(aq) Weak Bases- ammonium hydroxide, NH₂OH(aq) insoluble hydroxides such as Mg(OH)2(s), Al(OH)3(s), Fe(OH)3(s) Insoluble Salts*- silver chloride, AgCl(s) calcium carbonate, CaCO3(s) barium sulfate, BaSO4(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Completed and balanced c...View the full answer

Answered By

John Kimutai
I seek to use my competencies gained through on the job experience and skills learned in training to carry out tasks to the satisfaction of users. I have a keen interest in always delivering excellent work
4.70+
11+ Reviews
24+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Write a balanced net ionic equation for each of the following reactions: (a) Dilute nitric acid reacts with zinc metal with formation of nitrous oxide. (b) Concentrated nitric acid reacts with sulfur...
-
Write a balanced net ionic equation for each of the following solution reactions. Refer to Table 14.5 and Appendix D for electrolyte information. (a) Zn(NO 3 ) 2 (aq) + NaOH(aq) Zn(OH) 2 (s) + NaNO...
-
Write a balanced net ionic equation for each of the following solution reactions. Refer to Table 14.5 and Appendix D for electrolyte information. (a) AgNO 3 (aq) + KI(aq) AgI(s) + KNO 3 (aq) (b)...
-
Consider an atom diffuses in a 3 D simple cubic lattice by a random walk mechanism. The atom jumps 6 x 1 0 - 5 times per second at 3 0 0 K and 3 x 1 0 4 times per second at 6 0 0 K . Assuming that...
-
The exercise price on one of Flanagan Companys options is $15, its exercise value is $22, and its time value is $5. What are the options market value and the price of the stock?
-
The magnetic potential P at a point on the axis of a circular coil is given by where N, I, r, k, and c are constants. Find P. P= [" (1. 2 NIr k 1 (p + x) /2 dx
-
For each of the situations listed, identify the primary standard from the IMA Statement of Ethical Professional Practice that is violated (competence, confidentiality, integrity, or credibility)....
-
Given the minimal elements of a project audit present in Section 12.2, which element(s) would have been primary to the audit team? Why? Which section would have contained the underlying problems...
-
15. If y = 3x-4x+2 then slope at x = 1 is: (1) 1 (2) 2 (3) 4 (4) -2 16. sin240 = ? (1) - 2 (3) 3 1/2 17. Value of sin 2 is (4) (2) 12 (1) 23 (2) (3) 90 180 (4) 0 18. The rate of mass of the gas...
-
What term describes ions in a total ionic equation that do not react?
-
List the four steps for writing a balanced net ionic equation.
-
The equation cos 1 3x = 1 x has a root . a. Show, by calculation, that is between /15 and /12. b. Show that the given equation can be rearranged into the form with a suitable starting value, x 1 ,...
-
Can the original balanced scorecard by Kaplan and Norton be applied in a supply chain setting? Why or why not?
-
Explain which cost items cannot be easily summed up when measuring the performance of a whole supply chain (macro level).
-
Explain the concept of Value-at-risk (VaR) as a key risk measure. How is VaR typically calculated?
-
Explain the conditions for employing ABC in a supply chain network.
-
Why do an individual firms attempts to improve its C2C cycle not always lead to a corresponding improvement for the entire supply chain?
-
Prepare a flexible budget for 10,000, 12,000, and 14,000 units of output, using the following information: Variable costs Direct materials ...........$8.00 per unit Direct labor ............$2.50 per...
-
What are the two methods used to translate financial statements and how does the functional currency play a role in determining which method is used?
-
Make or buy unknown level of volume. (A. Atkinson) Oxford Engineering manufactures small engines. The engines are sold to manufacturers who install them in such products as lawn mowers. The company...
-
Make versus buy, activity-based costing opportunity costs. (N. Melumad and S. Reichelstein, adapted) The Ace Company produces bicycles. This years expected production is 10,000 units. Currently, Ace...
-
Multiple choice comprehensive problem on relevant costs. The following are the Class Companys unit costs of manufacturing and marketing a high-style pen at an output level of 20,000 units per month:...
-
How does a retail business design and implement a successful CRM program. What steps do they have to take to design the program?
-
CASE 4-1 A Nut Case The Molokai Nut Company (MNC) makes four different products from macadamia nuts grown in the Hawaiian Islands: chocolate-coated whole nuts (Whole), chocolate-coated nut clusters...
-
Job and career growth in the database industry are strong and predicted to remain strong over the next decade. Traditional database industry jobs terminate in the position of database administrator...

Study smarter with the SolutionInn App


