The van der Waals equation of state is where a and b are temperature-independent parameters that have
Question:
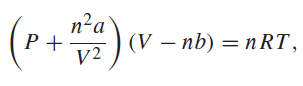
where a and b are temperature-independent parameters that have different values for each gas. For carbon dioxide, a = 0.3640 Pa m6 molˆ’2 and b = 4.267 × 10ˆ’5 m3 molˆ’1.
(a) Write this equation as a cubic equation in V.
(b) Use the NSolve statement in Mathematica to find the volume of 1.000 mol of carbon dioxide at P = 1.000 bar (100000 Pa) and T = 298.15 K. Two of the three roots are complex and must be ignored. Compare your result with the prediction of the ideal gas equation of state.
(c) Use the Find Root statement in Mathematica to find the real root in part b.
(d) Repeat part b for P = 10.000 bar (1.0000 × 106 Pa) and T = 298.15 K. Compare your result with the prediction of the ideal gas equation of state.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





