Consider two Boltzmannian gases (A) and (B), at pressures (P_{A}) and (P_{B}) and temperatures (T_{A}) and (T_{B}),
Question:
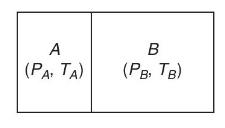
Consider two Boltzmannian gases \(A\) and \(B\), at pressures \(P_{A}\) and \(P_{B}\) and temperatures \(T_{A}\) and \(T_{B}\), respectively, contained in two regions of space that communicate through a very narrow opening in the partitioning wall; see Figure 6.8. Show that the dynamic equilibrium resulting from the mutual effusion of the two kinds of molecules satisfies the condition
\[
P_{A} / P_{B}=\left(m_{A} T_{A} / m_{B} T_{B}ight)^{1 / 2}
\]
rather than \(P_{A}=P_{B}\) (which would be the case if the equilibrium had resulted from a hydrodynamic flow).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





