Consider a nonrelativistic fluid that, in the neighborhood of the origin, has fluid velocity with ij
Question:
Consider a nonrelativistic fluid that, in the neighborhood of the origin, has fluid velocity
![]()
with σij symmetric and trace-free. As we shall see in Sec. 13.7.1, this represents a purely shearing flow, with no rotation or volume changes of fluid elements; σij is called the fluid’s rate of shear. Just as a gradient of temperature produces a diffusive flow of heat, so the gradient of velocity embodied in σij produces a diffusive flow of momentum (i.e., a stress). In this exercise we use kinetic theory to show that, for a monatomic gas with isotropic scattering of atoms off one another, this stress is
![]()
with the coefficient of shear viscosity

where ρ is the gas density, λ is the atoms’ mean free path between collisions, and
![]()
is the atoms’ rms speed. Our analysis follows the same route as the analysis of heat conduction.
(a) Derive Eq. (3.85b) for the shear viscosity, to within a factor of order unity, by an order-of-magnitude analysis.
(b) Regard the atoms’ distribution function N as being a function of the magnitude p and direction n of an atom’s momentum, and of location x in space. Show that, if the scattering is isotropic with cross section σs and the number density of atoms is n, then the Boltzmann transport equation can be written as
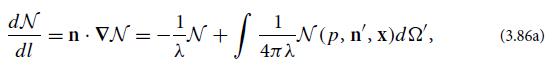
where λ = 1/nσs is the atomic mean free path (mean distance traveled between scatterings) and l is distance traveled by a fiducial atom.
(c) Explain why, in the limit of vanishingly small mean free path, the distribution function has the following form:
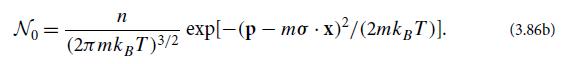
(d) Solve the Boltzmann transport equation (3.86a) to obtain the leading-order correction N1 to the distribution function at x = 0.
(e) Compute the stress at x = 0 via a momentum-space integral. Your answer should be Eq. (3.85a) with ηshear given by Eq. (3.85b) to within a few tens of percent accuracy.
Step by Step Answer:

Modern Classical Physics Optics Fluids Plasmas Elasticity Relativity And Statistical Physics
ISBN: 9780691159027
1st Edition
Authors: Kip S. Thorne, Roger D. Blandford





