A student synthesized Compound 1 (below). To purify the compound, he extracted it into aqueous base and
Question:
A student synthesized Compound 1 (below). To purify the compound, he extracted it into aqueous base and then acidified the solution to protonate the acid so that he could extract it back into ether. When he evaporated the ether, he found that his product had been converted entirely into Compound 2.
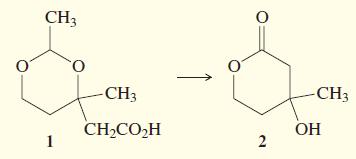
(a) What is the functional group that forms the ring in Compound 1? In Compound 2?
(b) How many carbon atoms are there in Compound 1? In Compound 2? Where did the other carbon atoms go?
(c) When did the reaction occur: When the student added the base, or when he added the acid?
(d) Propose a mechanism for the conversion of Compound 1 to Compound 2.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





