Some protecting groups can block two OH groups of a carbohydrate at the same time. One such
Question:
Some protecting groups can block two OH groups of a carbohydrate at the same time. One such group is shown here, protecting the 4-OH and 6-OH groups of -D-glucose.
(a) What type of functional group is involved in this blocking group?
(b) What did glucose react with to form this protected compound?
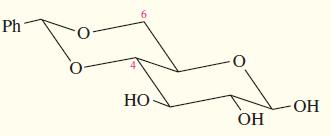
(c) When this blocking group is added to glucose, a new chiral center is formed. Where is it? Draw the stereoisomer that has the other configuration at this chiral center. What is the relationship between these two stereoisomers of the protected compound?
(d) Which of the two stereoisomers in part (c) do you expect to be the major product? Why?
(e) A similar protecting group, called an acetonide, can block reaction at the 2' and 3' oxygens of a ribonucleoside. This protected derivative is formed by the reaction of the nucleoside with acetone under acid catalysis. From this information, draw the protected product formed by the reaction.
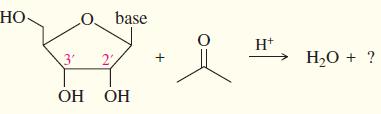
Step by Step Answer:






