(a) Explain why the SH stretching absorption in the IR spectrum of a thiol is less intense...
Question:
(a) Explain why the S—H stretching absorption in the IR spectrum of a thiol is less intense and occurs at lower frequency (2550 cm–1) than the O—H stretching absorption of an alcohol.
(b) Is the wavenumber difference between O—H and S—H absorptions caused primarily by the greater mass of sulfur or by the relative strengths of the two bonds? Explain how you know.
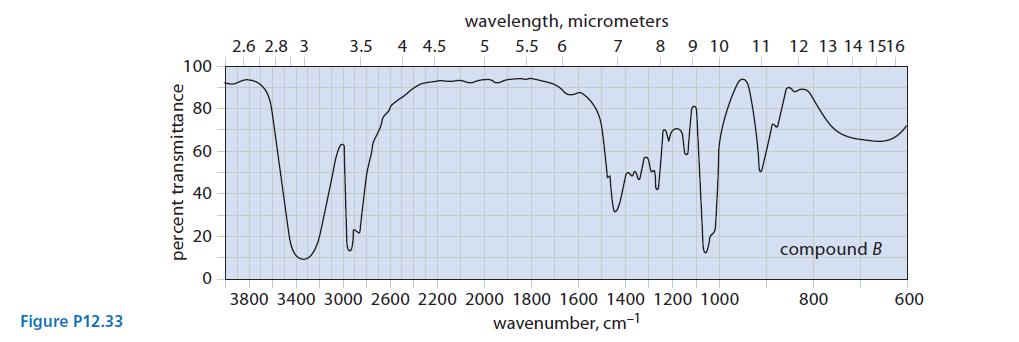
(c) Two unlabeled bottles, A and B, contain liquids. Laboratory notes suggest that one compound is (HSCH2CH2)2O and the other is (HOCH2CH2)2S.
The IR spectra of the two compounds are given in Fig. P12.33. Identify A and B and explain your choice.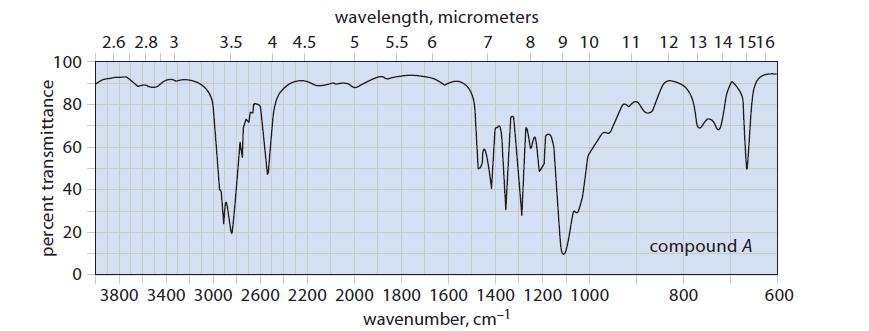
Transcribed Image Text:
100 percent transmittance 80 60 40 20 0 2.6 2.8 3 wavelength, micrometers 3.5 4 4.5 5 5.5 6 7 8 9 10 3800 3400 3000 2600 2200 2000 1800 1600 1400 1200 1000 wavenumber, cm-¹ 11 12 13 14 1516 compound A 800 600
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a b c The bond dipole of the SH bond is much less than that of the OH bond because the electroneg ativities of sulfur and hydrogen differ less than do ...View the full answer

Answered By

Hassan Imtiaz
The following are details of my Professional Experience. Responsibilities Eight years of demanding teaching experience in the field of finance and business studies at Master’s Level. Completion of the given tasks within given time with quality and efficiency. Marketing professional with practical experience in and solid understanding of a diverse range of management applications, including market analysis, sales and marketing, team building and quality assurance. I have excellent skills to approach deal and sustain corporate clients / customers by demonstrating not only extraordinary communication and interpersonal skills but also high caliber presentation, negotiation and closing skills. Manage and follow up the day-to-day activities. Manage and co-ordinate the inventories. Fulfillment of all the tasks assigned.
The following are details of my Areas of Effectiveness. Finance 1. Corporate Finance 2. Advanced Corporate Finance 3. Management of Financial Institutions 4. International Financial Management 5. Investments 6. Fixed Income 7. Real Estate Investment 8. Entrepreneurial Finance 9. Derivatives 10. Alternative Investments 11. Portfolio Management 12. Financial Statement Analysis And Reporting (US GAAP & IFRS) 13. International Financial Markets 14. Public Finance 15. Personal finance 16. Real estate 17. Financial Planning Quantitative Analysis 1. Time Value Of Money 2. Statistics 3. Probability Distribution 4. Business Statistics 5. Statistical Theory and Methods Economics 1. Principles of Economics 2. Economic Theory 3. Microeconomic Principles 4. Macroeconomic Principles 5. International Monetary Economics 6. Money and Banking 7. Financial Economics 8. Population Economics 9. Behavioral Economics International Business 1. Ethics 2. Business Ethics 3. An introduction to business studies 4. Organization & Management 5. Legal Environment of Business 6. Information Systems in Organizations 7. Operations Management 8. Global Business Policies 9. Industrial Organization 10. Business Strategy 11. Information Management and Technology 12. Company Structure and Organizational Management Accounting & Auditing 1. Financial Accounting 2. Managerial Accounting 3. Accounting for strategy implementation 4. Financial accounting 5. Introduction to bookkeeping and accounting Marketing 1. Marketing Management 2. Professional Development Strategies 3. Business Communications 4. Business planning 5. Commerce & Technology Human resource management 1. General Management 2. Conflict management 3. Leadership 4. Organizational Leadership 5. Supply Chain Management 6. Law 7. Corporate Strategy Creative Writing 1. Analytical Reading & Writing Other Expertise 1. Risk Management 2. Entrepreneurship 3. Management science 4. Organizational behavior 5. Project management 6. Financial Analysis, Research & Companies Valuation 7. And any kind of Excel Queries
4.80+
150+ Reviews
230+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
1. How strong are the competitive forces confronting J. Crew in the market for specialty retail? Do a [Michael Porter] five-forces analysis to support your answer. (see chapter 3 in the textfor...
-
Quiz# 5 (Q) The following are estimates for four risk assets (A,B,C,D). The portfolio P is an equal weighted portfolio of the four risk assets. Stock A Stock B Stock C Stock D Portfolio P weight 0.25...
-
What is the purpose of independent verification of performance?
-
Harrods PLC has a market value of 400 million and 30 million shares outstanding. Selfridge Department Store has a market value of 160 million and 18 million shares outstanding. Harrods is...
-
Zachary and Carrie Sexton (the Buyers) were searching for a home in the Kings wood neighborhood of Atlanta, Georgia. The Buyers real estate agent learned that Russell and Linda Sewell (the Sellers)...
-
Job costing, unit cost, ending work in process. Rafael Company produces pipes for concert quality organs. Each job is unique. In April 2011, it completed all outstanding orders, and then, in May...
-
Mr. Smith, a high school teacher in Gander, decided to quit his current job and opened a private tutorial firm. He gave up his $38,940 a year job as a teacher. He used $27,500 of his savings that...
-
Suggest structures for the following neutral molecules commonly lost in mass spectral fragmentation. (a) Mass = 28 from a compound containing only C and H (b) Mass = 18 from a compound containing C,...
-
Explain why a nitro compound has two NO stretching vibrations. (These typically occur at about 1370 and 1550 cm 1 .) R-N : :0: R-N :
-
Explain how to find sources of product differentiation.
-
In given list: [3, 9, 5, 4, 8, 1, 5, 2, 7, 6]. Apply heapify over this to make a min heap and sort the elements in decreasing order?
-
Converting Decimal Numbers to Binary Numbers using stack data structure. store reminders into the stack and then print the stack.
-
Find and analyze a contract that requires a co-signer.
-
As in Illustration 5.1-1 it is desired to produce liquefied methane; however, the conditions are now changed so that the gas is initially available at 1 bar and 200 K, and methane leaving the cooler...
-
The total inductance \(L_{\mathrm{P}}\) of a solid cylindrical conductor (of radius \(r\) ) due to both internal and external flux linkages out of distance \(\mathrm{D}\) is given by (in \(\mathrm{H}...
-
In 2008, Patricia purchases a rental property as an investment at a cost of $60,000. From 2008 through 2011, she takes $7,000 in depreciation on the property. In 2011, Patricia sells the rental...
-
When a company has a contract involving multiple performance obligations, how must the company recognize revenue?
-
1-Phenyl-2-butene has an ultraviolet absorption at max = 208 nm ( = 8000). On treatment with a small amount of strong acid, isomerization occurs and a new substance with max = 250 nm ( = 15,800) is...
-
What is the structure of a hydrocarbon that has M + = 120 in its mass spectrum and has the following 1 H NMR spectrum? 7.25 (5 H, broad singlet); 2.90 (1 H, septet, J = 7 Hz); 1.22 (6 H, doublet,...
-
Propose structures for compounds that fit the following descriptions: (a) C 10 H 14 H NMR: 7.18 (4 H, broad singlet); 2.70 (4 H, quartet, J = 7 Hz); 1.20 (6 H, triplet, J = 7 Hz) IR: 745 cm 1 (b)...
-
(b) The car dealership only sells cars that have fewer than 10000 miles and are 5 years old or less. (i) Write an algorithm that will: ask the user to enter the number of miles and the age of a car...
-
The following is given: Price $8.50/unit Variable cost $6.00/unit Fixed cost $56,000 a) Based on the given information, the break-even point in units units (enter your response as a whole number). =...
-
A firm has 43 units of a certain product on hand. Forecasts for the first two planning periods are 20 units each. A production quantity of 80 units is planned to be available in period 3. Customer...

Study smarter with the SolutionInn App


