(a) Give the simplified (nonaggregated) ionic structure of the enolate formed by treatment of 2,2-dimethylcyclohexanone with LDA;...
Question:
(a) Give the simplified (nonaggregated) ionic structure of the enolate formed by treatment of 2,2-dimethylcyclohexanone with LDA; then, give the product of its directed aldol reaction with acetaldehyde following acidic workup.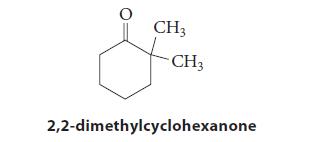
(b) Two diastereomers of the product in part (a) are formed. Explain. Are they formed in equal amounts or different amounts?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





