Account for the following observations with a mechanism. (1) In 80% aqueous ethanol, compound A reacts to
Question:
Account for the following observations with a mechanism.
(1) In 80% aqueous ethanol, compound A reacts to give compound B. Notice that trans-B is the only stereoisomer of this compound that is formed.
(2) Optically active A gives completely racemic B.
(3) The reaction of A is about 105 times faster than the analogous substitution reactions of both its stereoisomer C and chlorocyclohexane.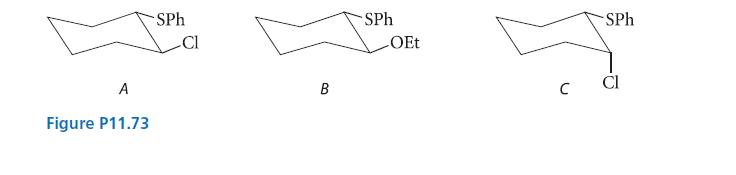
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





