Consider the following compounds and their dipole moments: Assume that the CCl bond dipole is oriented as
Question:
Consider the following compounds and their dipole moments: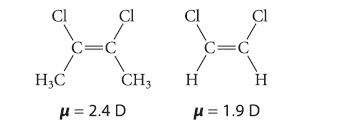
Assume that the C—Cl bond dipole is oriented as follows in each of these compounds.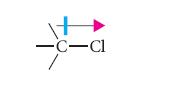
(a) According to the preceding dipole moments, which is more electron-donating toward a double bond, methyl or hydrogen? Explain.
(b) Which of the following compounds should have the greater dipole moment? Explain.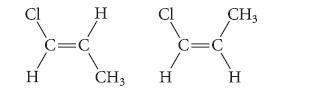
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





