For each of the following balanced oxidationreduction reactions, indicate which compound(s) are oxidized and which are reduced.
Question:
For each of the following balanced oxidation–reduction reactions, indicate which compound(s) are oxidized and which are reduced.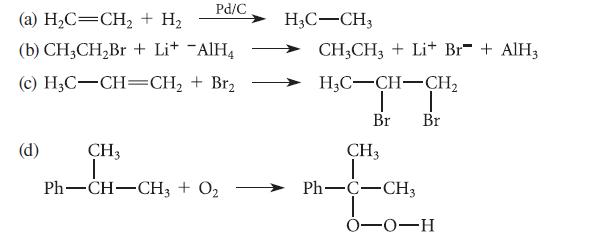
Transcribed Image Text:
Pd/C (a) H₂C=CH₂ + H₂ (b) CH3CH₂Br + Li+ AlH4 (c) H3C-CH=CH₂ + Br₂ (d) CH3 T Ph–CH–CH3 + O2 H3C-CH3 CH3CH3 +Li+ Br+AlH3 H3C-CH-CH₂ I T Br Br CH3 Ph—C–CH3 0-0-H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a The alkene is reduced and the H is oxidi...View the full answer

Answered By

Diane Joyce Pastorin
Please accept my enthusiastic application to solutioninn. I would love the opportunity to be a hardworking, passionate member of your tutoring program. As soon as I read the description of the program, I knew I was a well-qualified candidate for the position.
I have extensive tutoring experience in a variety of fields. I have tutored in English as well as Calculus. I have helped students learn to analyze literature, write essays, understand historical events, and graph parabolas. Your program requires that tutors be able to assist students in multiple subjects, and my experience would allow me to do just that.
You also state in your job posting that you require tutors that can work with students of all ages. As a summer camp counselor, I have experience working with preschool and kindergarten-age students. I have also tutored middle school students in reading, as well as college and high school students. Through these tutoring and counseling positions, I have learned how to best teach each age group.
4.60+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
KitchenPaid (KP) is a U.S. manufacturer of upscale small kitchen appliances. As a result of an acquisition made in 2013 KP also has a small division that manufactures medical supplies. KP is...
-
Each of the following reactions will be encountered at some point in this text. Classify each one according to whether the organic substrate is oxidized or reduced in the process. (a) (b) (c) (d) ...
-
Each of the following reactions will be encountered at some point in this text. Classify each one according to whether the organic substrate is oxidized or reduced in the process. (a) CH3C = PCH +...
-
Doug, Peter, and Jack have the following capital balances;$150,000, $300,000 and $320,000, respectively. The partners shareprofits and losses 35%, 40%, and 25% respectively. Jones is goingto pay a 2...
-
Refer to the data in PE 6-16. Calculate the companys average collection period. Data from PE 6-16 Accounts receivable balance, December 31 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ....
-
Champion had 2 million shares outstanding on December 31, Year 7, its year-end. On March 31, Year 8, Champion paid a 10% stock dividend. On June 30, Year 8, Champion sells $10 million of 5%...
-
Distinguish between direct and indirect environmental impacts of a division. Provide an example to illustrate the difference.Why is the difference important to a site manager?
-
Into what two categories does the FASAB divide government assets? How are each of the two accounted for?
-
How do pilots interpret sustainable practices to improve workforce development and diversity, increase quality and efficiency while reducing costs in aviation and aerospace operations.
-
Contrast the products expected when 2-methyl-3-pentanol is treated with (a) HBr/H 2 SO 4 or (b) Ph 3 PBr 2 . Explain.
-
Give the structure of two secondary alcohols that could be converted by HBr/H 2 SO 4 into the corresponding alkyl bromide without rearrangement.
-
Sketch the conic sections whose polar coordinate equations are given. Give polar coordinates for the vertices and, in the case of ellipses, for the centers as well. r || 2 1 + cos 0
-
A square loop of wire \(1.00 \mathrm{~m}\) on a side is at rest and has a linear charge density of zero in the Earth reference frame. The loop lies in the \(x y\) plane of an \(x y z\) coordinate...
-
When you unplug a coffee maker that plugs into the utility outlet of your car, you notice a spark. Worried that the coffee maker might be broken, you take it apart and find that the heating element...
-
A uniform magnetic field exists in a cubic volume of space with a \(50-\mathrm{mm}\) side length. If the magnetic energy stored in this volume is \(12 \mathrm{~J}\), what is the magnetic field...
-
Graph the relative frequency. Use the following information to answer question. Suppose Weight Watchers has collected the following weight loss data, in pounds, for 30 of its clients. 15, 20, 10, 6,...
-
Construct a frequency polygon for weight loss. Use the following information to answer question. Suppose Weight Watchers has collected the following weight loss data, in pounds, for 30 of its...
-
In your own words, describe the four types of quality-related activities.
-
What is the mode?
-
Write the equation for the final step in the -oxidation pathway of any fatty acid with an even number of carbon atoms.
-
Show the products of each of the followingreactions: (a) FAD FADH2 CHCH-CH2CH2CHzCsCOA Acyl-CoA dehydrogenase (b) Enoyl-CoA hydratase Product of (a) + H20 NAD+ NADH/H* (c) Product of (b)...
-
What is the structure of the a-keto acid formed by transamination of each of the following amino acids? (a) Threonine (b) Phenylalanine (c) Asparagine
-
Can a normal sized law firm handle this matter on our own or should they engage an Alternative Legal Service Provider? Why or why not? If the law frim was to engage an ALSP what kind of services...
-
Q3: Seasonality Analysis: Demand Month 2020 2021 January 1150 1250 2022 2023 1270 1120 February 1250 1400 1550 1350 March 1150 1200 1300 1370 April 1400 1450 1500 1105 May 1110 1250 1600 1350 June...
-
Q6: Capacity Planning - Capacity Levels (3 Marks) If you know that Zayed's branch operations at PRIMO's Pizza are designed to operate 16 hours a day. In addition, across the whole year, there will be...

Study smarter with the SolutionInn App


