In 1975, a report was published in which the reaction given in Fig. P9.85 was observed. TheOBs
Question:
In 1975, a report was published in which the reaction given in Fig. P9.85 was observed. The—OBs (brosylate) group is a leaving group conceptually like halide. (Think of this group as you would—Br.) Notice that the reaction conditions favor an SN2 reaction.
(a) This result created quite a stir among chemists because it seemed to question a fundamental principle of the SN2 reaction. Explain.
(b) Because the result was potentially very significant, the work was reinvestigated very soon after it was published.
In this reinvestigation, it was found that after about 10 hours’ reaction time, the product consisted almost completely of trans-P. Only on standing for a much longer time under the reaction conditions did cis-P form (and trans-P disappear) to give the product mixture shown in Fig. P9.85. Furthermore, when the trans isomer of S was subjected to the same conditions, mostly cis-P was formed after 10 h, but, after 5 days, the same 75:25 cis:trans product mixture was formed as in Fig. P9.85. Finally, subjecting pure cis-P or pure trans-P to the reaction conditions gave, after five days, the same 75:25 mixture. Explain these results.
(c) Why is cis-P favored in the product mixture?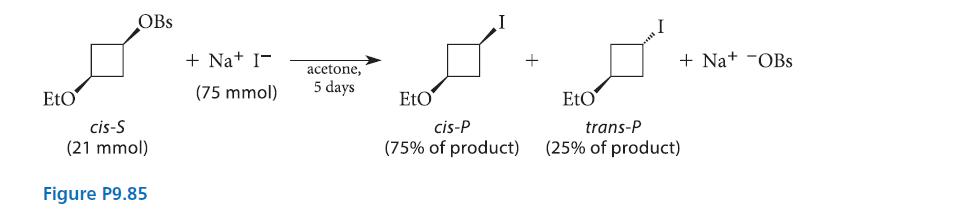
Step by Step Answer:






