In Fig. 25.2, the COP bond angle (118) suggests that the oxygen is sp 2 -hybridized. Use
Question:
In Fig. 25.2, the C—O—P bond angle (118°) suggests that the oxygen is sp2-hybridized. Use resonance structures to show why this hybridization and geometry is reasonable.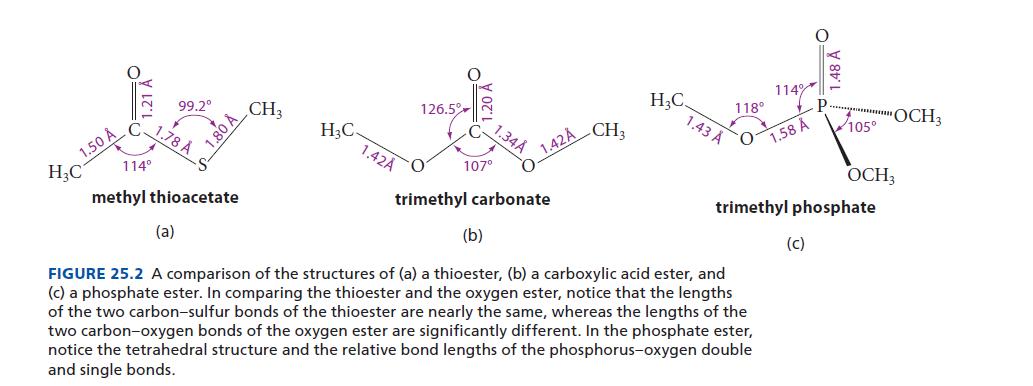
Transcribed Image Text:
1.50 Å H₂C 114⁰ 99.2° 1.78 Å (a) 1.80 Å methyl thioacetate CH3 H₂C. 1.42A 126.5% O 107° 1.34Å O 1.42Å trimethyl carbonate CH3 H₂C 1.43 Å 118⁰ O 114% (b) FIGURE 25.2 A comparison of the structures of (a) a thioester, (b) a carboxylic acid ester, and (c) a phosphate ester. In comparing the thioester and the oxygen ester, notice that the lengths of the two carbon-sulfur bonds of the thioester are nearly the same, whereas the lengths of the two carbon-oxygen bonds of the oxygen ester are significantly different. In the phosphate ester, notice the tetrahedral structure and the relative bond lengths of the phosphorus-oxygen double and single bonds. 1.58 A 105° trimethyl phosphate (c) OCH3 OCH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
If the oxygen were sphybridized then the COP bond ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use resonance structures to help you identify all sites of low electron density (δ+) in the following compound:
-
Use electron dot structures to show why tetramethylammonium hydroxide, (CH3)4N+OH-, is an ionic compound. That is, show why hydroxide is not covalently bound to the rest of the molecule.
-
Use resonance structures to help you identify all sites of high electron density (δ-) in the following compound:
-
Claud Chapperon is a self-employed distributor of wholesale clothing who began trading on 1 July 2012. His summarised accounts for the year to 30 June 2020 are shown below. The figures in brackets...
-
A sample of radioactive material is initially found to have an activity of 115.0 decays/min. After 4 d 5 h, its activity is measured to be 73.5 decays/min. (a) Calculate the half-life of the...
-
American Products is concerned about managing cash efficiently. On the average, inventories have an age of 90 days, and accounts receivable are collected in 60 days. Accounts payable are paid...
-
Let X X be a semi-martingale. Check that the solution of the S D E d S t = S t ( b ( t ) d t + ( t ) d X t ) S D E d S t = S t ( b ( t ) d t + ( t ) d X t ) is S t = S 0 exp ( U t ) t S t = S 0...
-
New Hampshire adopted a tax law that in effect taxed the income of nonresidents working in New Hampshire only. Austin, a nonresident who worked in New Hampshire, claimed that the tax law was invalid....
-
5. Consider the following weighted undirected graph as shown in Figure 3. Find the shortest path distance from node A to all other nodes. Show calculation of each iteration while finding the shortest...
-
The hydrolysis of phosphotyrosine esters in proteins is catalyzed by a family of enzymes called protein phosphotyrosine phosphatases. These hydrolyses in many cases involve phosphoenzyme...
-
Given the pK a values of methyl phosphate shown in this section, calculate the percentage of the un-ionized form, the monoanion form, and the di-anion form at pH 7.4.
-
Identify the four hierarchical levels used to classify costs. When can each of these levels of costs be avoided?
-
The International Monetary Fund (IMF) publishes World Economic Outlook Updates biannually. According to the July 2018 Update, the expected annual growth rate of advanced countries was 2.2% for 2019....
-
Why do people value liquidity? What are the different ways that banks, the bond market, and the stock market create liquidity?
-
For each of the following, use a graph to show the shift in aggregate demand. a. Poor numbers from several leading economic indicators cause businesses to become pessimistic about the future of the...
-
What do organizations need to do to make human-centered policies a priority for the future of work?
-
What are the two types of accounting exposure and how do they arise?
-
Find the probability in each case. a. N = 1000, n = 60, = 75, and = 6; P( < 76.5) =? b. N = 90, n = 36, = 108, and = 3.46; P(107 < < 107.7) =? c. N = 250, n = 100, = 35.6, and = 4.89; P( 36)...
-
Show that the block upper triangular matrix A in Example 5 is invertible if and only if both A 11 and A 22 are invertible. Data from in Example 5 EXAMPLE 5 A matrix of the form A = [ A11 A12 0 A22 is...
-
What product is formed when 2-methylpropanamide is subjected to the conditions of the Hofmann rearrangement in aqueous NaOH?
-
Show how 2-cyclopentyl-N,N-dimethylethanamine could be synthesized from each of the following starting materials: (a) (b) CH2 CN O- CH2 CHO (two ways)
-
Illustrate the Brgnsted basicity of Mescalino n.q by giving the structures of their conjugate acids,
-
Explain the following thoroughly for recitation guide purposes. 1. Distinction between Administrative Law and International Law ; Administrative Law versus Constitutional Law; Administrative Law vs...
-
a) Draw the pipeline execution diagram (similar to the diagram in slide 78 of the MIPS Microarchitecture lecture) of the following MIPS program. lw $s1, #4 ($s3) sub $t2, $s1, $t2 SW $t4, #4 ($s3)...
-
Two workers are sliding 280 kg crate across the floor. One worker pushes forward on the crate with a force of 390 N while the other pulls in the same direction with a force of 330 N using a rope...

Study smarter with the SolutionInn App


