Knowing that carbocations rearrange, chemists H. C. Brown and Glenn A. Russell at Purdue University in the
Question:
Knowing that carbocations rearrange, chemists H. C. Brown and Glenn A. Russell at Purdue University in the early 1950s investigated whether free-radical rearrangements might also occur: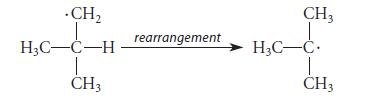
They carried out light-promoted free-radical chlorination of isobutane-2d, in which the tertiary hydrogen of isobutane was replaced by deuterium (2H = D), as shown in Fig. P9.81. The ratio of DCl to HCl produced in the reaction was found to be exactly the same as the ratio of A to B. (Any isotopically substituted A, if present, would not be differentiated from a.)
(a) This result was cited as evidence that free radicals do not rearrange.
(b) Assuming the same reaction conditions, how would the relative percentages of A and B compare with the percentages of tert-butyl chloride and isobutyl chloride in Eq. 9.88 (greater or less)? Explain.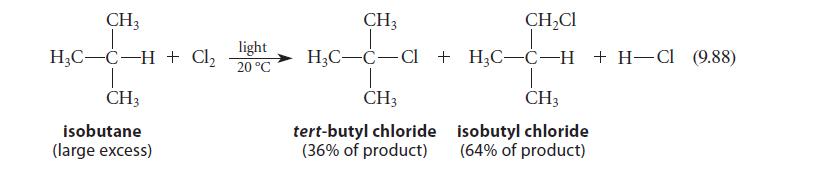
(c) Suggest a method for the preparation of the isotopically labeled starting material, isobutane-2d.
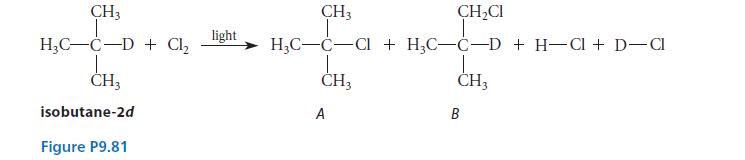
Step by Step Answer:






