Primary alcohols, when treated with H 2 SO 4 , do not dehydrate to alkenes under the
Question:
Primary alcohols, when treated with H2SO4, do not dehydrate to alkenes under the usual conditions. However, they do undergo another type of “dehydration” to form ethers if heated strongly in the presence of H2SO4. The reaction of ethyl alcohol to give diethyl ether is typical: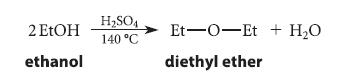
Using the curved-arrow notation, show mechanistically how this reaction takes place.
Transcribed Image Text:
2 EtOH ethanol H₂SO4 140 °C Et-O-Et + H₂O diethyl ether
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Ethanol is partially protonated under the acidic conditions Water is displaced from ...View the full answer

Answered By

Rinki Devi
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions.
Hi there! Are you looking for a committed, reliable, and enthusiastic tutor? Well, teaching and learning are more of a second nature to me, having been raised by parents who are both teachers. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students.
I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and helped them achieve great subject knowledge.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Do some amendment and enhance the given research paper: Table of Content Abstract..3 Action Research.4 Research Methodology and Design...5 Literature Review: NoSQL Database7 Proposal.7 Iteration 1..8...
-
Case Study: Quick Fix Dental Practice Technology requirements Application must be built using Visual Studio 2019 or Visual Studio 2017, professional or enterprise. The community edition is not...
-
TDS is a national delivery company based at Thornton Science Park. They employ 5 0 staff including administration, vehicle drivers, and warehouse workers. Their system is reliant on IT systems. Their...
-
The following accounts receivable information is for Kayley Company: Did the creditworthiness of Kayley's customers increase or decrease between 2010 and 2012?Explain. 2012 2011 2010 Accounts...
-
1. Review the concept of supply chain management. Although Passing Lane offers services rather than products, could the SCM concept apply to the design of the new system? Why or why not? 2. What...
-
Use information from Section 6.7 to estimate which form of electromagnetic radiation is the lowest energy ionizing radiation. Data from section 6.7 When we first introduced the concept of the...
-
Tidwell Corporation was organized on January 1, 2014. It is authorized to issue 20,000 shares of 6%, $50 par value preferred stock and 500,000 shares of no-par common stock with a stated value of $1...
-
Define and comprehend the concept of data base and its types. 7. Briefly discuss Database Management Systems. 8. Enumerate and briefly discuss the key concepts of Database Management Systems....
-
The reactions shown in Fig. P10.67 all involve conversion of the alcohol oxygen into a good leaving group, followed by the reaction of the resulting compound with a nucleophile provided by the...
-
Monoamine oxidase (MAO) is an enzyme that catalyzes the oxidation of certain biologically important amines. One form of the enzyme catalyzes the following oxidation of serotonin, an important...
-
Jane has 3 liters of soft drinks and 9 sandwiches. Bob, on the other hand, has 8 liters of soft drinks and 4 sandwiches. With these endowments, Janes marginal rate of substitution (MRS) of soft...
-
You ascertain that inventories and (to a lesser extent) trade receivables have risen significantly when you consider that sales have increased by only 5%. Discuss the questions that you ask and the...
-
Calculate the van der Waals parameters from critical point data for the following gases: He, CH4, NH3, and H2O. Explain the relative magnitudes of a and b from a physical basis.
-
Assume that money demand takes the form \[ \frac{M}{P}=Y\left[1-\left(r+\pi^{e}ight)ight] \] where \(Y=1,000\) and \(r=0.1\). a. Assume that, in the short run, \(\pi^{e}\) is constant and equal to...
-
Compute the two-year nominal interest rate using the exact formula and the approximation formula for each set of assumptions listed in (a) through (c). a. \(i_{t}=2 \% ; i_{t+1}^{e}=3 \%\) b....
-
For which of the problems listed in (a) through (c) would you want to use real payments and real interest rates, and for which would you want to use nominal payments and nominal interest rates to...
-
Sterling is a college professor with an extensive stock portfolio. Last year, he met Wheeler, a stockbroker with the firm of Ransom, LaForge, and Adkins. To get Sterlings business, Wheeler offered to...
-
Solve each problem. Find the coordinates of the points of intersection of the line y = 2 and the circle with center at (4, 5) and radius 4.
-
Show the mechanism of the nucleophilic addition reaction of an alcohol with an iso-cyanate to yield a urethane.
-
What product would you expect to obtain from catalytic hydrogenation of natural rubber? Would the product be syndiotactic, atactic, or isotactic?
-
Propose a mechanism to account for the formation of Bakelite from acid-catalyzed polymerization of phenol and formaldehyde.
-
a) Write a suitable C function to satisfy the following: i. The name of the function is community. ii. When it is called, it will return a floating point number. iii. The function would accept 3...
-
john's experience with prentice hall's lauching an $800 accounting software package was: a complete disaster or due partially to his sales predictions and lauch decisions or a lesson in how to...
-
Do you have to join a union to work in production accounting in the entertainment industry?

Study smarter with the SolutionInn App


