The following compound is a very strong base; its conjugate acid has a pK a of about
Question:
The following compound is a very strong base; its conjugate acid has a pKa of about 13.5. Give the structure of its conjugate acid and show that it is stabilized by resonance.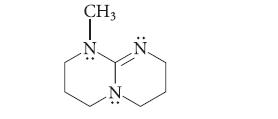
Transcribed Image Text:
CH3 -Z:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
The conjugate acid is formed by prot...View the full answer

Answered By

Shristi Singh
A freshman year metallurgy and material science student in India.
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
1. What segment of the external environment has more impact on an organization? 2. If an organization wants to engage in a new industry, which area of the industry environment should be the most...
-
The following compound is a suicide inhibitor of the enzyme that catalyzes amino acid racemization: Propose a mechanism that explains how this compound irreversibly inactivates the enzyme. HC CCHO NH
-
Three bottles A, B. and C have been found, each of which contains a liquid and is labeled "amineC8H11N." As an expert in amine chemistry, you have been hired as a consultant and asked to identify...
-
Consider the utility function U(x 1 , x 2 ) = x 1 x 2 with budget constraint p 1 x 1 + p 2 x 2 = c. (a) Show that the maximum of U(x 1 , x 2 ) subject to the budget constraint is equal to c 2 /(4p 1...
-
Explain how organizations in the not-for-profit sector differ from organizations in the public sector or for-profit business sector. Provide an example of an entity in each sector.
-
Shown below is a segmented income statement for Hickory Companys three wooden flooring product lines: Hickorys parquet flooring product line has a contribution margin of $50,000 (sales of $300,000...
-
Marissa's spaceship approaches Joseph's at a speed of \(0.99 c\). As Marissa passes Joseph, they synchronize their clocks to both read \(t=0 \mathrm{~s}\). When Marissa's clock reads \(100...
-
Foxy Investigative Services is an investigative services firm that is owned and operated by Shirley Vickers. On November 30, 2018, the end of the fiscal year, the accountant for Foxy Investigative...
-
Explain the purpose of preparing financial statements for non-reporting entities in Australia. You must address: Financial transparency Stakeholder communication Compliance with regulatory...
-
Which should be more reactive in nitration: -picoline or -picoline? Explain using resonance structures, and give the major nitration product(s) in each case.
-
Draw the structure of the major form of each of the following compounds present in an aqueous solution containing initially one molar equivalent of 1 M HCl. Explain your reasoning. (a) quinine (Fig....
-
Carey exchanges real estate for other real estate in a qualifying like-kind exchange. Careys basis in the real estate given up is $120,000, and the property has a fair market value of $165,000. In...
-
Which tax service is known for its willingness to take a stand on controversial issues not covered by legislation or tax law?
-
Pam recently married Henry. When she was single she used a calendar tax year. For a variety of reasons she is considering changing her tax year. When would she not need IRS permission to change her...
-
How early can amounts be distributed from a qualified plan penalty-free?
-
When might a taxpayer prefer a sale over a like-kind exchange that would result in nonrecognition of gain under Section 1031?
-
Which of the following taxes are deductible for federal income tax purposes as an itemized deduction? a. Ad valorem personal property tax b. FICA tax imposed on employees c. Federal gift tax d. Sales...
-
Determine the equation of the regression line for the following data, and compute theresiduals. 15 19 12 y 47 36 56 44 21
-
Why is homeostasis defined as the "relative constancy of the internal environments? Does negative feedback or positive feedback tend to promote homeostasis?
-
Show how one can work backwards from the target step-by-step to the "present situation" in each of the following real-life problems. Target: Successfully financing a college education. Present...
-
Give the product expected, if any, when 2- methyl-2- propanol (or other compound indicated) reacts with each of the following reagents. (a) concentrated aqueous HCI (b) CrO3, in pyridine (c) H2SO4,...
-
Give the structure of a compound that satisfies the criterion given in each case. (There may be more than one correct answer.) An alcohol that, after acid-catalyzed dehydration. yields an alkene...
-
Find the capitalization error. An error can be a word that is not capitalized and should be, or an error can be a word that is capitalized and should not be. Let's invite Mr. roth to our cookout on...
-
Sheldon has automobile insurance with ABC Insurance Company. During an icy day, Sheldon's car slid, bounced over a curb and landed against a hydro pole. The hydro pole was damaged, requiring the...
-
Suppose Hagadorn Venture Capital makes a $5M Series A investment in NewTech for 1M shares at $5 per share. One year later, NewTech has fallen on hard times and receives a $5M Series B financing from...

Study smarter with the SolutionInn App


