The N-methylquinolinium ion forms a noncovalent complex with molecule A in water that has a standard free
Question:
The N-methylquinolinium ion forms a noncovalent complex with molecule A in water that has a standard free energy of dissociation ΔG°d 5 28.9 kJ mol–1 (6.9 kcal mol–1). The neutral molecule 4-methylquinoline forms a noncovalent complex with molecule A in water with ΔG°d 5 22.2 kJ mol–1 (5.3 kcal mol–1).
(a) Calculate the dissociation constant for each complex.
(b) Suggest a reason that the binding of the ion to A is stronger than the binding of the neutral molecule to A.
(c) The N-methylquinolinium ion forms a noncovalent complex with molecule B with ΔG°d = 35.2 kJ mol–1 (8.4 kcal mol–1). Suggest a reason that the complex with molecule B has a smaller dissociation constant than the complex with molecule A.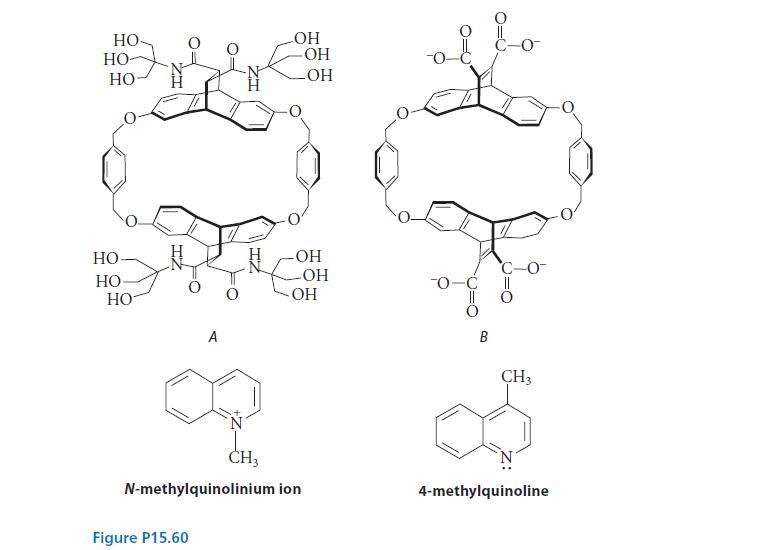
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





