The principles for predicting bond angles do not permit a distinction between the following two conceivable forms
Question:
The principles for predicting bond angles do not permit a distinction between the following two conceivable forms of ethylene.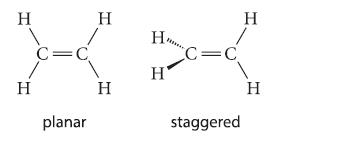
The dipole moment of ethylene is zero. Does this experimental fact provide a clue to the preferred dihedral angles in ethylene? Why or why not?
Transcribed Image Text:
H H C=C planar H H H₂ Н' C=C staggered H H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
No matter how any CH group is turned the resultant bo...View the full answer

Answered By

Gilbert Chesire
I am a diligent writer who understands the writing conventions used in the industry and with the expertise to produce high quality papers at all times. I love to write plagiarism free work with which the grammar flows perfectly. I write both academics and articles with a lot of enthusiasm. I am always determined to put the interests of my customers before mine so as to build a cohesive environment where we can benefit from each other. I value all my clients and I pay them back by delivering the quality of work they yearn to get.
4.80+
14+ Reviews
49+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The principles for predicting bond angles do not permit a distinction between the following two conceivable forms of ethvlene. The dipole moment of ethylene is zero. Does this experimental fact...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Fresh" is a fresh fruit shopping chain. Their specialty is organically grown and seasonal fruits. They now operate about 10 outlets in Pune. Fruits are obtained from farmers within and near-by states...
-
Jin, the recruitment manager at Randents Inc., reviews the performance of his team members on a monthly basis. Based on the results of his monthly reviews, he decides to conduct daily reviews to...
-
How do business writers organize most informational reports, and what can writers assume about the audience?
-
Refer to problem 55. Yohanse uses the warehouse for 4 years and sells it. During this period, he properly deducts a total of $25,000 in depreciation. What is Yohanses gain or loss on the warehouse if...
-
Discuss the themes, theory, and/or phenomenon that would be anticipated to emerge as a result of the examination. Develop a hypothetical research scenario that would necessitate the use of the Action...
-
Jake Miille has just joined the Ciudad Juarez factory (text example) as the new production manager. He was pleased to see the company uses activity-based costing. Miille believes he can reduce...
-
23 Consider the following Java code: What will be printed when the following code is executed: new Cat().eat(); ? public class Animal ( } public void eat () { System.out.println("Animal is eating");...
-
Three possible dihedral angles for H 2 O 2 (0, 90, and 180) are shown in Fig. 1.6. (a) Assume that the H 2 O 2 molecule exists predominantly in one of these arrangements. Which of the dihedral angles...
-
Account for the fact that H 3 CCl (dipole moment 1.94 D) and H 3 CF (dipole moment 1.82 D) have almost identical dipole moments, even though fluorine is considerably more electronegative than...
-
Mack Darin owns and operates a store in a rough downtown neighbourhood. Last week, he received a shipment of switchblade knives. He placed one of the knives in his store window, alongside a small...
-
A department's staff has changed as follows over the last five years: During year 1, staff increased 20%. During year 2, staff decreased 25%. During year 3, staff increased 40%. During year 4, staff...
-
CHECK YOUR UNDERSTANDING 214 21 L01-1 9. Why is it important to start saving early to meet your short-term and long-term needs? hier not insmi LO1-2 10. Explain the concept of compounding and how...
-
How is the disposal group of a long - lived asset held for sale reported ? Long - lived assets held for sale are reported with other long - lived assets . Assets and liabilities of the disposal group...
-
One hundred students are writing the final exam in Dr. Strangepork's course on statistics. The mean score on the final exam is 54 with a standard deviation of 8. Treat these as population parameters....
-
At its core, an Appreciative Inquiry worldview would stand in stark opposition to unethical practice, which could explain why scarcely little is available in print that discusses the need for ethical...
-
Explain how marketable emission credits add to overall economic efficiency, compared to across-the-board limitations on maximum discharges of air pollutants by firms.
-
In the figure, two loudspeakers, separated by a distance of d1 = 2.63 m, are in phase. Assume the amplitudes of the sound from the speakers are approximately the same at the position of a listener,...
-
Show the products of thesereactions: . Cl Br . b) , a) CH;CH,CH,CH, + OH CI c) CH2=CHCH, +
-
Explain why only one of the two chlorines of 1, 2-dichloro-2-methylpropane is replaced by a hydroxy group when the compound is heated in water (see the preceding hydrolysis reaction.
-
On the basis of the bond cleavage shown for this reaction in Figure 10.1, predict the stereo chemistry of the product.Explain. OCCH, CH,CH, ." -
-
In a paragraph or so, briefly explain the shortcommings of the state's regulative role when it comes to employment matters governed by labour law.?
-
Module is called Labour Law (CLA 3651). Please make reference to each case as you explain With reference to Cymot (Pty) Ltd v McLaoud 2002 NR 391 (LC)); Labour Supply Chain v Hambata 2012 (1) NR 313...
-
Please advise on the labour laws and industrial relations system in Singapore ?

Study smarter with the SolutionInn App


