This problem refers to the reactions shown in Eqs. 3.12a and 3.12b. When equal numbers of moles
Question:
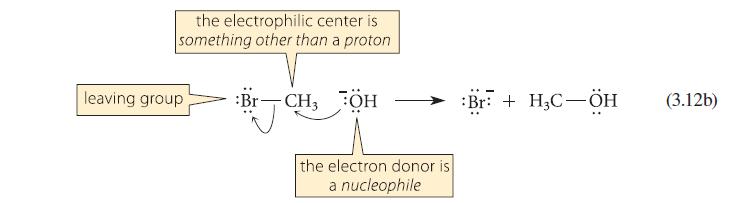
This problem refers to the reactions shown in Eqs. 3.12a and 3.12b. When equal numbers of moles of –OH, H—Br, and H3C—Br, are placed in solution together, what products are formed?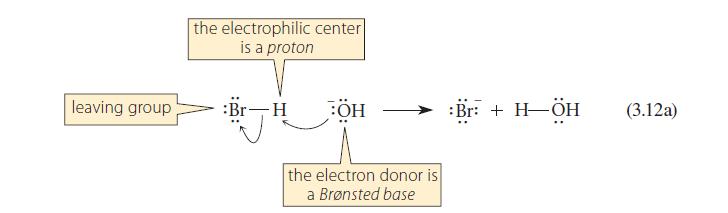
Transcribed Image Text:
leaving group the electrophilic center is a proton :Br-H BÖH the electron donor is a Brønsted base :Br: + H-ÖH (3.12a)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
As noted on text p 98 Brnsted acidbase reactions are g...View the full answer

Answered By

Anoop V
I have five years of experience in teaching and I have National Eligibility in teaching (UGC-NET) .
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
This problem refers to the radial keratotomy study data from Problem 12 in Chapter 8. Suppose that we want to compare the average change in refraction for males and females, controlling for baseline...
-
This problem refers to the 1990 Census data presented in Problem 19 of Chapter 5. In addition to median selected monthly ownership costs (OWNCOST), another independent variable studied was the...
-
This problem refers to the 1990 Census data presented in Problem 19 of Chapter 5 and in Problem 14 of Chapter 8. Use the computer output from Problem 14 of Chapter 8, along with the additional output...
-
Describe, in human terms, why delay and jitter are bad in real time (interactive) voice and video communications. Would these same problems apply to recorded voice and video stored and played back at...
-
How is a House-Senate conference committee different from other committees?
-
What is a Pareto chart? What is its function?
-
Several professionals are listed as being part of compliance efforts with regard to participation in the examination of potential compliance violations. Identify at least three other categories of...
-
Danny Badens Verde Vineyards in Oakville, California, produces three varieties of wine: Merlot, Viognier, and Pinot Noir. His winemaster, Russel Hansen, has identified the following activities as...
-
1. Explain why all of the following values are equal. What is the value shown in each line below? 9-2 9-x-y 1 dzdydx 9-x2-y2 /9-y2 9-y2-22 2 1 dxdzdy 9-y2 8 3 9-x c9-xz 70 1 dydzdx
-
A histidine residue (B), one of the functional groups in the structure of a certain enzyme, has a conjugate-acid pK a = 7.8. What is the fraction of each form (BH and B) present at physiological pH...
-
For each of the following electron-pair displacement reactions, give the curved-arrow notation; identify the nucleophile, the nucleophilic center, the electrophile, the electrophilic center, and the...
-
In Exercises 17 through 32, sketch the graph of the given function. f(x) = x 5 5x 4 + 93
-
Many feel that Small Businesses are at a significant disadvantage when competing with large national chains. Do you share this opinion? In what ways can a Small Business successfully compete with a...
-
1. Under traditional common law, in accepting an offer, may an offeree requestadditional terms? 2. What differences does the UCC make to the common law "mirror image" rule?
-
A portfolio has 75 shares of Stock A that sell for $33 per share and 110 shares of Stock B that sell for $26 per share. What is the portfolio weight of Stock A? Portfolio weight What is the portfolio...
-
What are the environmental and lifestyle factors that influence hormone levels and hormone-dependent processes, including diet, exercise, circadian rhythms, and exposure to endocrine-disrupting...
-
1-What is the difference between the Civil Law System and the Common Law System? Explain your answer. Is Saudi ArabiaaCivil Law System or a Common Law System? and Why? 2-Explain the Characteristics...
-
Briefly explain the use of graphs as a way to represent economic relationships. What is an inverse relationship? How does it graph? What is a direct relationship? How does it graph? Graph and explain...
-
Data on weekday exercise time for 20 females, consistent with summary quantities given in the paper An Ecological Momentary Assessment of the Physical Activity and Sedentary Behaviour Patterns of...
-
When dissolved in water, trichloroacetaldehyde (chloral, CCl3CHO) exists primarily as chloral hydrate, CCl3CH (OH)2, better known as knockout drops. Show the structure of chloral hydrate.
-
The oxygen in water is primarily (99.8%) 16O, but water enriched with the heavy isotope 18O is also available. When an aldehyde or ketone is dissolved in 18O-enriched water, the isotopic label...
-
Cyclohexanone forms cyanohydrins in good yield but 2, 2, 6-trimethylcyclo-hexanone does not. Explain.
-
Answer the following questions on data collection methods. (a) Describe two advantages a personal interview would have over telephone interviews and self-administered survey questionnaires. (b)...
-
List four main differences between document Nosql database and key-value NoSql database ?
-
Imagine somebody is working on a project that previously used relational databases. Draft a report to their client that explains why they should consider NoSQL databases. Remember that their clients...

Study smarter with the SolutionInn App


