Use their structures and dielectric constants to classify each of the following substances according to their solvent
Question:
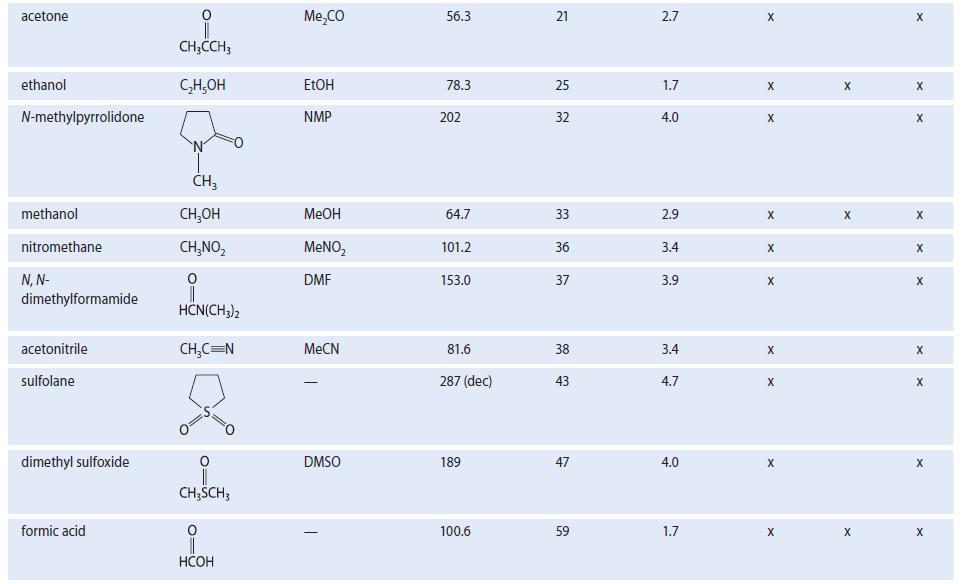
Use their structures and dielectric constants to classify each of the following substances according to their solvent properties (as in Table 8.2).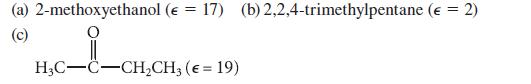

Transcribed Image Text:
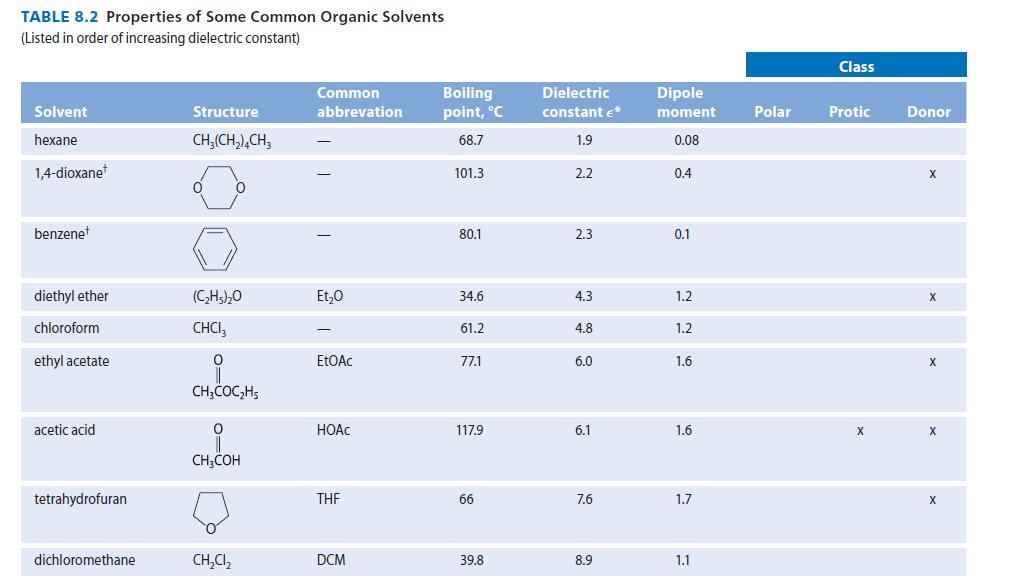
(a) 2-methoxyethanol (E = 17) (b) 2,2,4-trimethylpentane (€ = 2) (c) H3C-C-CH₂CH3 (€ = 19)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To classify the given substances according to their solvent properties we can use their dielectric c...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
When you are selecting a norm group, what ethical and legal concerns have to be addressed with each person participating? does this vary if they are adults or children?
-
Classify each of the following substance according to their solvent properties (as in Table 8.2.) (a) 2,2,2-triftuoroethanol ( - 26) (b) 2,2,4-trimethylpentane ( : 2)
-
Classify each of the following substances as a nonelectrolyte, weak electrolyte, or strong electrolyte in water: (a) H2SO3 (b) C2H5OH (ethanol) (c) NH3 (d) KClO3 (e) Cu(NO3)2.
-
.How dependent do you think the success of zero-based budgeting would be on the types of attribution models used? How might you increase the odds of success in this budgeting approach? .Advertising,...
-
In some carpet factories, children as young as 5 years old are shackled to looms and forced to work. They receive little food and no pay. How can their parents permit this to happen?
-
Marjorie is single and has the following investment income: Interest on savings ..... $2,900 Municipal bond interest .... 1,500 Dividends .......... 7,600 She pays investment interest expense of...
-
1. Your instructor will divide the class into teams and assign each team the task of investigating the start-up of one of the following businesses: a. Submarine sandwich shop b. Day care service c....
-
Tarmac Chemical Corporation produces a special chemical compoundcalled CHEMIXthat is used extensively in high school chemistry classes. This compound must contain at least 20% sulfur, at least 30%...
-
The schedule below produces same outcome as the serial schedule < T1, T5>. But it is not view serializable. Why? T read (A) A := A-50 write (A) read (B) B := B+50 write (B) TS read (B) B: B-10 write...
-
If the sawtooth pattern of melting point behavior is explained by the efficiency of crystal packing, what would we expect to find if we were to plot the density of the crystal (solid) form of...
-
Match the structures with the following melting points. Explain. 168172C; 74.5C; 143147C; 157C. (The alkenes are liquids at room temperature, and the carboxylic acids are solids.) HC HC A CH3 CH3 COH...
-
Calculate the net after-tax cash flow effect of the following information: sales, $260; expenses other than depreciation, $140; depreciation expense, $50; marginal income tax rate, 35%.
-
The average time on hold when calling technical support for an internet provider is customer will be kept on hold less than 4.0 minutes. = 5.2 minutes. Find the probability that a ift 0 HINT: use the...
-
1. A 15-year 6.5% coupon (semiannual) bond sells for $965, what is the bond holder's YTM? Please explain your answer in detail and how you would solve this answer step by step using a financial...
-
Principles of Process Scheduling a. Explain the different approaches to process scheduling (example - backward scheduling, forward scheduling, etc) and provide an example of how each can be applied....
-
After the light in Sarah's room was out, Tricia and Benji sat up talking about the evening's events. "I really am sorry that my plan to show Sarah you can be trusted backfired so badly, and made you...
-
Suppose the price of a can of soda in France is 1.50. Knowing that the nominal euro/dollar exchange rate is 1 = $1.40, how many dollars will a can of soda cost the American tourist on vacation in...
-
Identify a specific example of each of the following in this chapters Last Word: (a) Entrepreneurship, (b) Invention, (c) Innovation, (d) Diffusion.
-
Les has collected stamps in his spare time for years. He purchased many of his stamps at a price much lower than the current market value. Les recently lost his job as a carpenter. Since his wife...
-
Suggest a structure for the compound with the formula C5H7NO2 that has the following IR and 1H-NMR spectra. C;H;NO, 80- 60 ET 40 20- 0- 1740 cm-1 4000 3500 3000 2500 2000 1500 1000 500 Wavenumber...
-
Suggest a structure for the compound with the formula C5H10O2 that has the following IR and 1H-NMR spectra (some absorptions overlap in the NMRspectrum) C,H,02 80 60 40- 20 1733 cm- 1000 500 1500...
-
An unknown compound, A (C7H10), show four absorptions in its 13C-NMR spectrum, at 22 (CH2), 24, 124 (CH), and 126 (CH) on reaction with excess H2 and a Pt catalyst, A produces B (C7H14) B shows a...
-
A. Show that no anx" n=0 Let {an} is a sequence, with lim, an converges for || < 1 and lim (1 - 2) x-1- = anx" = A.
-
11 --6) is an eigenvector of the matrix A-1, where the matrix Compute all possible values of k. 2 1 1 --(1) A= 1 2 1 1 1 2 Suppose the vector
-
A roller coaster car starts from the top of a hill (a), 16 m high. The car then gradually coasts along the path (b) to the bottom (c) and up to the top of the adjacent hill (d), 9 m high. Ignoring...

Study smarter with the SolutionInn App


