What is the oxidation state of the metal in the starting material in the following reaction? How
Question:
What is the oxidation state of the metal in the starting material in the following reaction? How does it change, if at all, as a result of the reaction? Is this reaction an oxidation, a reduction, or neither?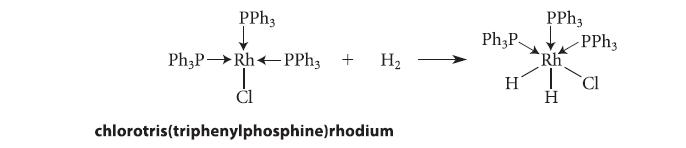
Transcribed Image Text:
PPh3 Ph3P→→Rh PPh3 + H₂ I CI chlorotris(triphenylphosphine) rhodium Ph3P H PPh3 Rh H -PPh3 CI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
With one Xtype ligand CI and zero charge oxidation state of rhodiu...View the full answer

Answered By

Caroline Kinuthia
Taking care of the smaller details in life has a larger impact in our general well being, and that is what i believe in. My name is Carol. Writing is my passion. To me, doing a task is one thing, and delivering results from the task is another thing. I am a perfectionist who always take things seriously and deliver to the best of my knowledge.
4.90+
1933+ Reviews
4269+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Suppose that a life aged 30 arranged an insurance with the following parameters. If death occurs in the first 20 years, 10,000 is paid. Otherwise, 20,000 is paid. Moreover, it was arranged with the...
-
Consider a thin square sheet of side L and thickness t, made of a material of resistivity p. The resistance between two opposite faces, shown by the shaded areas in the figure is A) directly...
-
You have decided to purchase additional memory for your computer in order to better support the latest version of the Windows operating system. At the local computer store, you notice that not only...
-
Consider the generalized externality problem. Assume the damage and cost functions are given by: (a) Determine the non-regulated level of E if the polluter has the right to pollute. (b) Determine the...
-
The mean lifetime of a pion traveling at high speed is measured to be 7.5 10 -8 s. Its lifetime when measured at rest is 2.6 10 -8 s. How fast is the pion traveling?
-
What is reliable accounting information? Identify and define the ingredients of reliable accounting information.
-
What is the distinction between the ready and waiting states of process scheduling?
-
Holism Consulting is a consulting firm owned and operated by Scott Cutler. The end-of period spreadsheet shown below was prepared for the year ended May 31, 2014. Based on the preceding spreadsheet,...
-
Accounting for salaries expense without using a reversing entry requires the following on the date of payment in the next period: Multiple choice question. compound entry that debits the expense and...
-
(a) What is the electron count for the Rh complex shown in Problem 18.9c? (b) Sestamibi (trade name Cardiolite ) is a complex of 99 Tc(I) (a radioactive -ray emitter) that is widely used for cardiac...
-
Calculate the oxidation state of the metal in each of the following complexes. (a) O Mn-O- permanganate (b) Pd(PPH3)4 tetrakis(triphenylphosphine) palladium (c) CpFe ferrocene
-
Of the first 10,000 votes cast in an election, 5,180 were for candidate A. Find a 95% confidence interval for the fraction of votes that candidate A will receive.
-
Can you think of another medical designation for which a regression discontinuity technique might be useful?
-
If emergency rooms decided to only serve people with health insurance, regardless of their condition, what would you expect that to do to the problem of adverse selection in the health insurance...
-
In academic disciplines where few women earn a Ph.D., there are considerably fewer women professors. How does this affect the gender imbalance in these fields?
-
Many colleges offer pass/fail classes. Use the ideas of adverse selection and moral hazard to explain why teachers in these classes find that pass/fail students rarely score at the top of the class.
-
Explain how imperfect information problems such as adverse selection and moral hazard might affect the following markets or situations: a. The market for wireless internet connectivity at large...
-
Use Table A.2, Appendix A, to find the values of the following binomial distribution problems. a. P(x = 14 | n = 20 and p = .60) b. P(x < 5 | n = 10 and p = .30) c. P(x 12 | n = 15 and p = .60) d....
-
During registration at Tech every quarter, students in the Department of Management must have their courses approved by the departmental advisor. It takes the advisor an average of 4 minutes...
-
Tri-butyltin hydride (Bu3SnH) is used synthetically to reduce alkyl halides, replacing a halogen atom with hydrogen. Free-radical initiators promote this reaction, and free-radical inhibitors are...
-
When healthy, Earth's stratosphere contains a low concentration of ozone (O3) that absorbs potentially harmful ultraviolet (UV) radiation by the cycle shown at right. Chlorofluorocarbon refrigerants,...
-
Deuterium (D) is the hydrogen isotope of mass number 2, with a proton and a neutron in its nucleus. The chemistry of deuterium is nearly identical to the chemistry of hydrogen, except that the C-D...
-
How do viruses exhibit remarkable genetic diversity and adaptive potential through mechanisms such as mutation, recombination, and reassortment, and what are the implications of viral genetic...
-
You've collected the following information from your favorite financial website. 52-Week Price Dividend PE Close Hi 77.40 Lo 10.43 Stock (Dividend) Yield % Ratio Price Net Change Acevedo .36 2.6 6...
-
Provide answer for the following question with an example 1. Explain why supply chain risk, vulnerability, robustness and resilience have emerged as important themes in LSCM. 2. Identify the sources...

Study smarter with the SolutionInn App


