When 1-buten-3-yne undergoes HCl addition, two compounds A and B are formed in a ratio of 2.2
Question:
When 1-buten-3-yne undergoes HCl addition, two compounds A and B are formed in a ratio of 2.2 : 1. Neither compound shows a C ≡ C stretching absorption in its IR spectrum. Compound B reacts with maleic anhydride to give compound C, and compound A undergoes allylic rearrangement to compound B on heating. Propose structures for compounds A and B and explain your reasoning.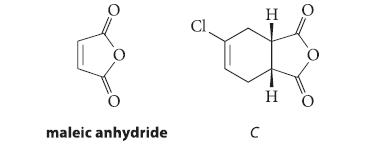
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





