When the alcohol A undergoes acid-catalyzed dehydration, two isomeric alkenes are formed: B and C (see Fig.
Question:
When the alcohol A undergoes acid-catalyzed dehydration, two isomeric alkenes are formed: B and C (see Fig. P15.67a). The relative percentage of each alkene formed is shown as a function of time in Fig. P15.67b. The composition of the alkene mixture at very long times is the equilibrium composition. Furthermore, if either alkene is subjected to the conditions of the reaction, the equilibrium mixture of alkenes is obtained.
(a) Is the dehydration a kinetically controlled or thermodynamically controlled reaction? Explain.
(b) Give a structural reason why compound C is favored at equilibrium.
(c) Suggest one reason why alkene B is formed more rapidly.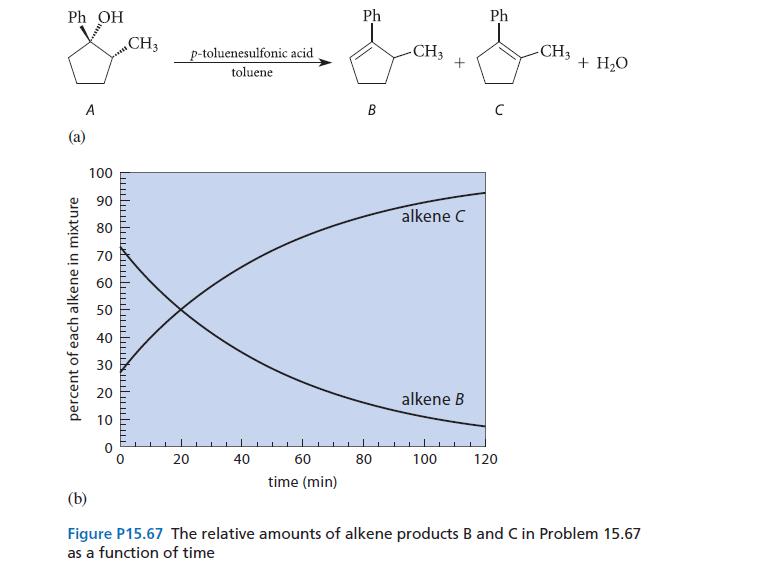
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





