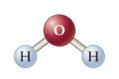
As Figure P23.43 shows, the water molecule is bent, and the angle formed by the three atoms
Question:
As Figure P23.43 shows, the water molecule is bent, and the angle formed by the three atoms is \(104.5^{\circ}\). Given that the permanent dipole moment of the molecule is \(6.186 \times 10^{-30} \mathrm{C} \cdot \mathrm{m}\), determine the dipole moment of each \(\mathrm{O}-\mathrm{H}\) bond.
Data from Figure P23.43
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





