Consider an ideal gas undergoing the two processes depicted in Figure (mathbf{2 0 . 3 7}) between
Question:
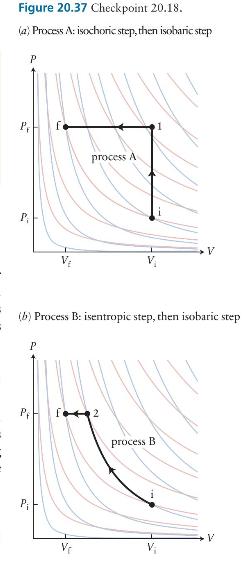
Consider an ideal gas undergoing the two processes depicted in Figure \(\mathbf{2 0 . 3 7}\) between the same initial and final states that lie on an isotherm. In process \(A\) the gas is taken via an isochoric process to an intermediate state 1 and then via an isobaric process to the final state. In process B the gas undergoes an isentropic transformation to an intermediate state 2 and then an isobaric transformation to the final state. Which process has the greater value for \((a)\) the work done on the gas and \((b)\) the magnitude of the energy transferred thermally to the gas?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





