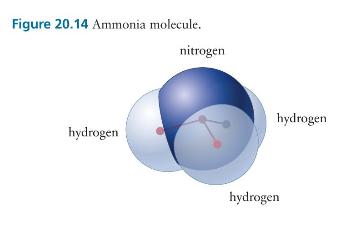
Consider the four-atom ammonia molecule illustrated in Figure 20.14. (a) Which of the three rotational degrees of
Question:
Consider the four-atom ammonia molecule illustrated in Figure 20.14.
(a) Which of the three rotational degrees of freedom can store thermal energy?
(b) Based on your answer to part \(a\), what is the thermal energy per molecule?
(c) What is the heat capacity per molecule?
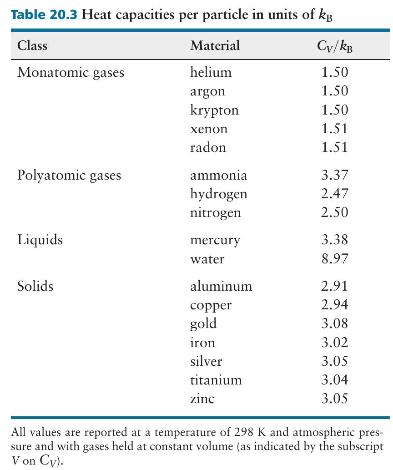
(d) Does your prediction in part \(c\) agree with the experimental value listed in Table 20.3?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





