A gas stream contains 18.0 mole% hexane and the remainder nitrogen. The stream flows to a condenser,
Question:
A gas stream contains 18.0 mole% hexane and the remainder nitrogen. The stream flows to a condenser, where its temperature is reduced and some of the hexane is liquefied. The hexane mole fraction in the gas stream leaving the condenser is 0.0500. Liquid hexane condensate is recovered at a rate of 1.50 L/min.
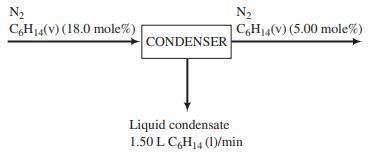
(a) What is the flow rate of the gas stream leaving the condenser in mol/min? (First calculate the molar flow rate of the condensate and note that the rates at which C6H14 and N2 enter the unit must equal the total rates at which they leave in the two exit streams.)
(b) What percentage of the hexane entering the condenser is recovered as a liquid?
(c) Suggest a change you could make in the process operating conditions to increase the percentage recovery of hexane. What would be the downside?
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





