a. In this chapter, the assumption was made that the harmonic oscillator model is valid such that
Question:
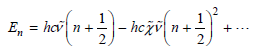
Neglecting zero-point energy, the energy levels become En = hcν̅n €“ hcχ̅ ν̅n2 +...
Using the preceding expression, demonstrate that the vibrational partition function for the anharmonic oscillator is
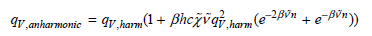
In deriving the preceding result, the following series relationship will prove useful:
b. For €“1 H2, ν̅ = 4401.2 cm and χ̅ ν̅ = 121.3 cm€“1. Use the result from part (a) to determine the percent error in qV if anharmonicity is ignored.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





