A small pharmaceutical firm plans to manufacture a new drug and has hired you as a consultant
Question:
A small pharmaceutical firm plans to manufacture a new drug and has hired you as a consultant to design a condenser to remove the drug from a gas–vapor mixture. The mixture, which contains 20 mole% of the drug and the balance nitrogen, will be fed to the condenser at 510K and 1 atm at a rate of 3.5 L/s. Of the drug fed to the unit, 90% must be condensed. No physical property data are available for the drug, and part of your job is to acquire the data needed to design the condenser. The company has sent you a large sample of the liquid drug for this purpose.
You acquire an insulated 2.000-liter container with a known heat capacity and a built-in electrical heating coil that can deliver a known heat input to the contents of the container. A calibrated thermocouple is used to measure the temperature in the vessel, and the pressure is measured with a mercury manometer.
You carry out a series of experiments on a day when atmospheric pressure is 763mm Hg.
Experiment 1.
Fill the container with the liquid, then seal and weigh.
mass of container + liquid = 4:4553 kg
mass of evacuated container = 3:2551 kg
Next, starting at each of two temperatures (T0), add a fixed quantity of heat to the liquid, observe the final temperature (Tf ), and subtract the heat absorbed by the container from the total heat input to determine the amount of the heat added to the liquid, Qa.
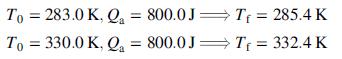
Assume that the liquid heat capacity may be expressed as a linear function of temperature (Cv = aT + b) when analyzing these results.
Experiment 2.
Pour a small quantity of the drug into the container, place the container in a liquid nitrogen bath to freeze the drug, evacuate all of the air, and seal the container. Weigh the container after it comes back to room temperature.
mass of container + drug = 3:2571 kg
Next heat the sealed container until all of the liquid evaporates, and repeat Experiment 1.

Assume that the vapor heat capacity may be expressed as a linear function of temperature when analyzing these results.
Experiment 3.
Fill approximately half the container with the drug, freeze, evacuate the air, and seal. Measure the pressure at several temperatures, verifying that liquid is present in the container at each temperature.
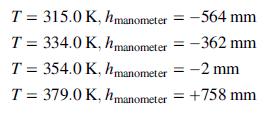
(a) Using the given data, determine the following physical properties of the drug: (i) liquid specific gravity, (ii) molecular weight, (iii) linear expressions for the heat capacities at constant volume [in J/(mol K)] for both the liquid and vapor [Cv = a + bT(K)], (iv) linear expressions for Cp for both liquid and vapor, (v) a Clausius–Clapeyron expression for p*(T), (vi) the normal boiling point, and (vii) the heat of vaporization (in J/mol) at the normal boiling point.
(b) Calculate the required condenser temperature, assuming operation at 1 atm.
(c) Calculate the rate at which heat must be removed in the condenser, taking the heat capacity of nitrogen to be constant at 29.0 J/(mol K).
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





