A useful strategy for the study of electron transfer in proteins consists of attaching an electroactive species
Question:
A useful strategy for the study of electron transfer in proteins consists of attaching an electroactive species to the protein’s surface and then measuring ket between the attached species and an electroactive protein cofactor. J.W. Winkler and H.B. Gray (Chem. Rev. 92, 369 (1992)) summarize data for cytochrome c modified by replacement of the haem iron by a zinc ion, resulting in a zinc–porphyrin (ZnP) group in the interior of the protein, and by attachment of a ruthenium ion complex to a surface histidine amino acid. The edge-to-edge distance between the electroactive species was thus fixed at 1.23nm. A variety of ruthenium ion complexes with different standard potentials was used. For each ruthenium-modified protein, either the Ru2+→ZnP+ or the ZnP*→Ru3+, in which the electron donor is an electronically excited state of the zinc–porphyrin group formed by laser excitation, was monitored. This arrangement leads to different standard reaction Gibbs energies because the redox couples ZnP+/ZnP and ZnP+/ZnP* have different standard potentials, with the electronically excited porphyrin being a more powerful reductant. Use the following data to estimate the reorganization energy for this system:
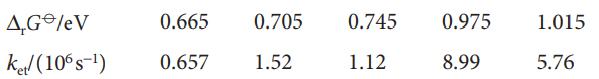
Step by Step Answer:

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula





