As mentioned in the text, the viscosity of liquids decreases with increasing temperature. The empirical equation η
Question:
a. How can one use the equation provided to determine A and E given a series of viscosity versus temperature measurements?
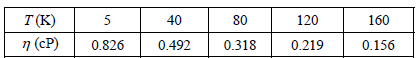
b. Use your answer in part (a) to determine A and E for liquid benzene given the following data:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





