Calculate the standard enthalpy of formation of FeS 2 (s) at 600. °C from the following data
Question:
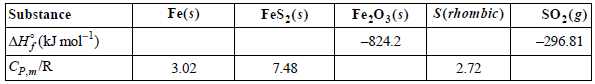
You are also given that for the reaction 2FeS2(s) + 11/2O2(g) †’ Fe2O2(s) + 4SO2(g).ˆ†HoR = - 1655 kJ mol-1
Transcribed Image Text:
Substance S(rhombic) so, (g) FeS:(s) Fe(s) Fe,03(s) -824.2 AH, (kJ mol-) -296.81 3.02 7.48 2.72 CP,/R
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
2FeS 2 s 112O 2 gFe 2 O 2 s 4SO 2 gH o R 1655 kJ mol 1 1655 kJ mo...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the standard enthalpy of formation of NOCI (g) from the enthalpy of formation of NO given in Table 2.5, together with the following information: 2 NOCl (g) 2 NO (g) + Clz (g) 1 Ho = + Uo=...
-
The molar heat capacity of ethane is represented in the temperature range 298 K to 400 K by the empirical expression Cp,m/ (J K-1 mol-1) = 14.73 + 0.1272(T/K). The corresponding expressions for C(s)...
-
The head of a strike anywhere match contains tetraphosphorus trisulfide, P4S3. In an experiment, a student burned this compound in an excess of oxygen and found that it evolved 3651 kJ of heat per...
-
A factor used in measuring the loudness sensed by the human ear is (I/I 0 ), 0.3 where I is the intensity of the sound and I 0 is a reference intensity. Evaluate this factor for I = 3.2 10 6 W/m 2...
-
Write a short "employee Code of Conduct".
-
A steel rod of \(25 \mathrm{~mm}\) diameter is placed concentrically in a copper tube of an internal diameter \(38.5 \mathrm{~mm}\) and external diameter of \(41.5 \mathrm{~mm}\). Nuts and washers...
-
Suppose you are working with a data set that has some very "different" (much larger or much smaller than the rest of the data) scores. What measure of central tendency would you use and why?
-
Kras, Inc., was organized and authorized to issue 50,000 shares of $100 par value, 9 percent [referred stock and 50,000 a shares of no-par, $5 stated value common stock on July 1, 2011. Stock-related...
-
How collaborative leadership can help principals balance discipline with other responsibilities? How would you intentionally structure your leadership teams to address instructional leadership?
-
The June 30 unadjusted trial balance of Prime Realty appears as follows: Additional Information: 1. Rent expires at a rate of $700 per month. 2. Monthly depreciation on equipment is $300. 3. Interest...
-
Count the total number of s bonds and p bonds in the compound below: -3-N
-
Use the tabulated values of the enthalpy of combustion of benzene and the enthalpies of formation of CO 2 (g) and H 2 O(l) to determine H o f for benzene.
-
Explain how ABC can help a firm identify its true low-cost suppliers.
-
Purple Corporation is undergoing financial difficulty and planning to liquidate its assets. Current ratio is currently at 1.5x. Purple Corp. current liabilities is 500,000; non-current liabilities of...
-
The following financial information is given. Year 1 Year 2 Book value of assets $18,000 $26,000 Market value of equity 18,000 60,000 12 months ended Year 1 12 months ended Year 2 Sales $1,000 $1,300...
-
Briefly describe tax administrative matters including: Tax collection and withholding mechanisms (PAYG withholding, PAYG instalments).
-
In 20X1, Lee was hurt in the course of his employment with Foster Farms. In 20X1, Lee was paid the following amounts from Foster Farms' worker's compensation insurance company: $30,000 for a physical...
-
S&J Catering is a small catering business operating in Western Sydney. Business partners, Jack and Simon, established the business a year ago. To start up the business, the partners contributed...
-
Find the values of the variables for which each statement is true, if possible. [x y z] = [21 6]
-
The production budget of Artest Company calls for 80,000 units to be produced. If it takes 30 minutes to make one unit and the direct labor rate is $16 per hour, what is the total budgeted direct...
-
Calculate the magnitude of the diffusion-controlled rate constant at 298 K for a species in (a) Decylbenzene, (b) Concentrated sulfuric acid. The viscosities are 3.36 cP and 27 cP, respectively.
-
Calculate the magnitude of the diffusion-controlled rate constant at 298 K for the recombination of two atoms in benzene, for which 17 = 0.601 cP. Assuming the concentration of the reacting species...
-
For the gaseous reaction A + B --7 P, the reactive cross-section obtained from the experimental value of the pre-exponential factor is 8.7 X 10-22 nm. The collision cross-sections of A and B...
-
Popular furniture company, IKEA, has purchased forests in Romania as well as land in Alabama to assist with keeping up with the wood demand necessary to complete customer orders. This was one way...
-
How does China being Turkey's biggest import partner affect Turkey's exchange rate?
-
Assignment 4 In this assignment you are provided information on an experiment and you are required to investigate and interpret the output which is provided below. Problem: Consider the...

Study smarter with the SolutionInn App


