Chemists recognize that the cyclohexyl radical is likely to be more stable than the cyclopentylmethyl radical, because
Question:
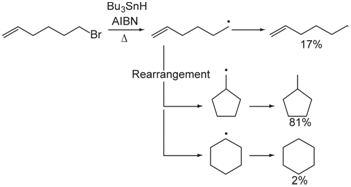
The two possible interpretations for the experimental result are that the reaction is thermo-chemically controlled but that our understanding of radical stability is wrong or that the reaction is kinetically controlled.
a. First, see if you can rule out the first possibility. Examine structures and total energies for cyclohexyl and cyclopentylmethyl radicals (€œcyclohexyl, cyclopentylmethyl radicals€ on the precalculated Spartan file). Which radical, cyclohexyl or cyclopentylmethyl, is more stable (lower in energy)? Is the energy difference large enough such that only the more stable radical is likely to be observed? (Recall that at room temperature an energy difference of 12 kJ/mol corresponds to a product ratio of > 99:1.) Do you conclude that ring closure is under thermodynamic control?
b. The next objective is to establish which ring closure, to cyclohexyl radical or to cyclopentylmethyl radical, is easier; that is, which product, cyclohexane or methylcyclopentane, is the kinetic product? Examine structures and total energies for the transition states for the two ring closures (€œto cyclohexyl and cyclopentylmethyl radicals€ on the Spartan download). Which radical, cyclohexyl or cyclopentylmethyl, is more easily formed?
c. Consider the following relationships between transition-state energy difference, ΔE€¡, and the ratio of major to minor (kinetic) products, calculated from the Boltzmann distribution:
ΔE€¡ (kJ/mol)______________________Major: Minor (room temperature)
4€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦. ˆ¼90:10
8€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦. ˆ¼95:5
12€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦€¦. ˆ¼99:1
What is the approximate ratio of products suggested by the calculations? How does this compare with what is observed? Do you conclude that ring closure is under kinetic control?
Step by Step Answer:






