Consider the comparison made between accurate results and those based on calculations using the van der Waals
Question:
Figure 7.1
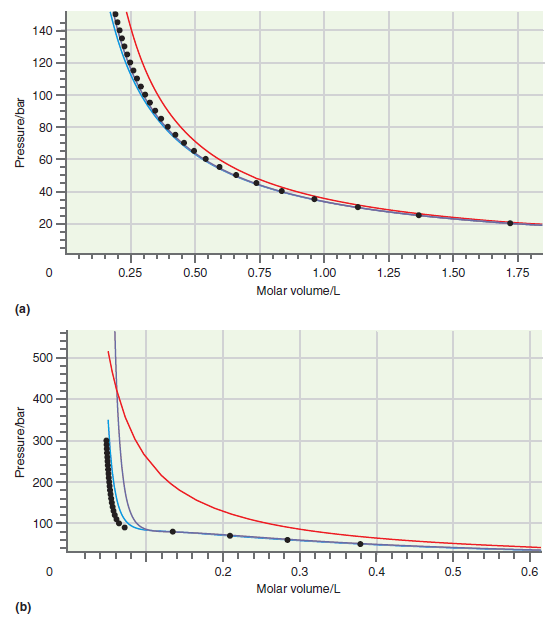
Figure 7.5
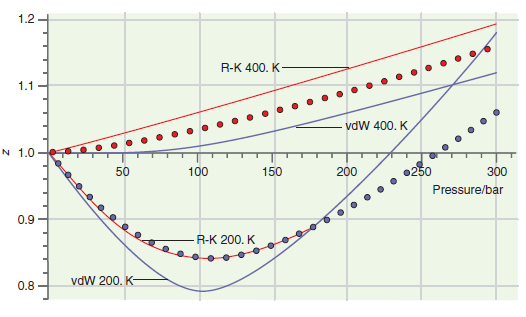
Transcribed Image Text:
140 120 100 80 40 20 0.25 0.75 0.50 1.00 1.25 1.50 1.75 Molar volume/L (a) 500 400 300 200 100 0.2 0.3 0.4 0.5 0.6 Molar volume/L (b) Pressure/bar Pressure/bar 1.2 R-K 400. K 1.1 vdW 400. K N 1.0 100 150 200 •250 300 Pressure/bar 0.9 R-K 200. K vdW 200. K- 0.8
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (15 reviews)
The RedlichKwong gives more ac...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Explain why the oscillations in the two-phase coexistence region using the RedlichKwong and van der Waals equations of state (see Figure 7.4) do not correspond to reality. Figure 7.4 140 120 100 304...
-
The van der Waals equation of state, an approximate representation of the behavior of gases at high pressure, is given by Where a and b are constants having different values for different gases. (In...
-
Derive the following relation, for the internal pressure of a gas that obeys the RedlichKwong equation of state, aU av /T 2TV, + b) || RT 1 V , + b)
-
1. As shown by point D in Fig 3.1, the volume of an ideal diatomic gas is 2.00L at standard condition (STP, T=273.15K, P=101.3kPa). The gas is heated to A with its volume conserved, expands...
-
Select a region of the world. Research current events and discuss how economic, political, and social changes will impact growth in the region. Write a short paper summarizing your findings.
-
In the Lucid / CCIV case, suppose that after the deal was announced, negative information were to come out about Lucids future prospects. As a result, the PIPE investors renegotiate their terms to...
-
Have world poverty and instability ended?
-
Jupiter Company sells goods on January 1 that have a cost of $500,000 to Danone Inc. for $700,000, with payment due in 1 year. The cash price for these goods is $610,000, with payment due in 30 days....
-
Choose a planned learning activity for a group of children and select and prepare the resources required for the activity or explain and list all resource you will use. b) Explain in detail the...
-
A ship is pulled at a constant speed by two small boats, A and B, as shown. The engine of the ship does not produce any force. The tension in each cable between A and B and the ship is 4000 N. a....
-
We return to the 60. kg hiker of P4.34, who is climbing the 828 m tall Burj Khalifa in Dubai. If the efficiency of converting the energy content of the bars into the work of climbing is 25%, the...
-
The initiation step for radical addition of HBr is highly endothermic: (a) Explain how this step can be thermodynamically favorable at high temperature even though it is endothermic. (b) Explain why...
-
Read the referenced article that fully describes the OR study summarized in the application vignette presented in Sec. 18.5. Briefly describe how inventory theory was applied in this study. Then list...
-
2. When preparing different types of meet cuts how would you prepare a rack of lamb using Frenching technique
-
3 Jamie is a shareholder in Trolley Corporation. In the current year, Jamie receives 500 non-taxable shares of common stock from Trolley Corp. that are identical to the 270 shares of Trolley Corp....
-
In the Cuckoo's Egg, Cliff started his initial investigation by: In the Cuckoo's Egg, Cliff started his initial investigation by: auditing all root level accounts disabling Sventek account analyzing...
-
A factory is experiencing a reduction in sales that has lasted an extended period of time due to a recession. With inventory piling up in the warehouse, the factory should send everyone home until...
-
ABC Company is experiencing financial difficulties due to a down economy. As a result, XYZ Bank requested that ABC Company pledge additional collateral to secure a line of credit necessary to fund...
-
Identify and sketch the graph of each relation. (x + 7) 2 + (y - 5) 2 + 4 = 0
-
2.) Find the Laplace transform of f(t) 7e-St cos 2t +9 sinh2 2t. Use Laplace Table. %3D
-
Suppose that a molecular orbital has the form N (O.l45A + 0.844B). Find a linear combination of the orbitals A and B that is orthogonal to this combination.
-
Show that, if a wave cos kx centred on A (so that x is measured from A) interferes with a similar wave cos k' centred on B (with x measured from B) a distance R away, then constructive interference...
-
Before doing the calculation below, sketch how the overlap between a is orbital and a 2p orbital can be expected to depend on their separation. The overlap integral between an H Is orbital and an H2p...
-
Differentiate between a scalar value and a vector value.
-
Now that the supplier of the nori has been chosen and the transportation method selected, you turn your attention to the downstream side of the supply chain -- the marketing (distribution) channels....
-
Mandy Murphy, owner of Murphy and Co. (Saint John, New Brunswick), gave the following list of assets and liabilities to a public accountant and asked him to prepare a balance sheet for the company as...

Study smarter with the SolutionInn App


